2. 中心实验室(上海市, 200040)
2. Central Laboratory, Shanghai Children's hospital, Shanghai Jiao Tong University, Shanghai 200040, China
隐睾是小儿泌尿外科的常见病,睾丸引带发育异常是隐睾发生的重要因素之一[1]。睾丸引带由Hunter[2]在1762年命名,是牵引睾丸下降的条索状结构。研究表明,在动物及人类胚胎期的睾丸引带内均存在肌肉组分[4, 5]。已有动物研究发现睾丸引带的成肌分化异常与隐睾的发生有关[3];在临床上人类低位隐睾患儿的引带中也发现横纹肌成分,且经HCG治疗后其引带组织结构重塑,表现为横纹肌体积含量较未治疗的患儿明显增加[4]。但是,高位隐睾的引带中是否有肌肉组分目前尚未见文献报道。另外,Soito等[5]在人类高位隐睾及低位隐睾中发现睾丸引带细胞外基质成分中总胶原成分存在差异。结合上述研究结果,我们有理由提出,人类高低位隐睾引带组织的结构和功能不尽相同,人类的睾丸引带发育,尤其是肌肉或肌肉样分化的异常可能导致睾丸下降异常。因此,本实验拟通过对人类高位及低位隐睾的睾丸引带进行组织学切片染色,观察其组织构成,以探寻人类隐睾睾丸引带组织中肌肉成分与高、低位隐睾的关系,以期为睾丸引带与人类隐睾的病因学研究提供组织学线索,为激素治疗低位隐睾提供一定解剖学依据。
材料与方法 一、研究材料1.标本来源:人类睾丸引带组织来源于上海市儿童医院泌尿外科的隐睾患儿, 患儿术前均明确诊断为隐睾(n=73),且未伴发其他泌尿生殖系统畸形,未接受内分泌治疗及其他促进睾丸下降的治疗。根据麻醉后患儿未降睾丸的位置分为两组:①高位组(n=11):位于内环口以上腹腔内体检未触及隐睾;②低位组(n=62):出内环口但是未出外环口的隐睾。本实验招募高位组患儿11例,年龄为0.9~1.7岁,中位年龄1.1岁;招募低位组患儿62例;年龄为0.8~4.3岁,中位年龄1.3岁;两组患儿年龄比较差异无统计学意义(P>0.05)。本研究已通过上海市儿童医院伦理委员会的批准(编号:2016R009-F01),标本采集均获得患儿家长的同意。
2.实验材料:多聚甲醛(PFA)、二甲苯、乙醇、石蜡、HE及Masson试剂、中性树脂、胎牛血清、柠檬酸盐、0.3%PBT、抗体MY-32(ab51263)、荧光二抗、DAPI试剂、封片剂。
二、研究方法1.组织切片染色:将术中所取的睾丸引带组织浸入10%PFA中固定24 h,依次梯度酒精脱水,二甲苯透明,浸蜡,包埋,切片(横断面),二甲苯脱蜡,乙醇浸水,分别进行HE及Masson(丽春红-酸性品红-苯胺蓝)染色,乙醇脱水,二甲苯透明,中性树脂封片。
2.免疫荧光染色:将术中所取的睾丸引带组织浸入10%PFA中固定24 h,依次梯度酒精脱水,二甲苯透明,浸蜡,包埋,切片(横断面),二甲苯脱蜡,乙醇浸水,抗原修复,漂洗3次,10%胎牛血清封闭30 min,MY-32抗体孵育过夜(4 ℃),漂洗3次,室温孵育二抗30 min,漂洗3次,DAPI染核,封片,荧光显微镜观察。
三、统计学处理采用SPSS 23.0统计软件进行统计学分析。对于年龄等符合正态分布的计量资料采用(x±s)表示,两组间比较采用独立样本t检验;对于高低位组是否能观察到横纹肌结构等计数资料采用频数分析,两组间比较采用Fisher's确切概率法。以P < 0.05为差异有统计学意义。
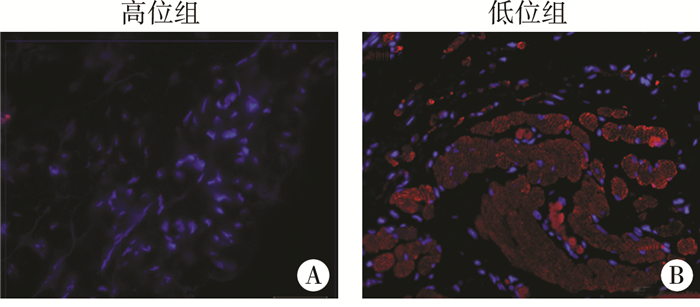
结 果 一、HE染色与Masson染色人类高位隐睾引带组织HE染色,可见其主要为较致密的结缔组织,含较多成纤维细胞、少量小血管,未见横纹肌组织结构(图 1A、图 1a);低位隐睾引带HE染色,则表现为混合性组织,可见较疏松排列的成纤维细胞、少量血管和较多纤维,可观察到横纹肌(图 1B、图 1b);高位引带Masson染色可见其组织中结构较致密,含有大量胶原纤维成分(蓝色)和少量小血管(紫红色)(图 1C、图 1c);低位引带Masson染色可见疏松的胶原纤维成分、小血管、横纹肌结构(图 1D、图 1d)。横纹肌特异性免疫荧光MY-32染色在高位组未观察到阳性信号(图 2A),低位组中可见明显横纹肌结构(图 2B)。

|
Download:
|
| 图 1 高低位隐睾引带组织结构 注 图A~D为200倍视野;图a~d为400视野 Fig. 1 Histological structure of gubernaculum testis from high/low cryptorchid children | |
|
Download:
|
| 图 2 高低位隐睾引带组织MY-32免疫荧光染色(×400) 注 A:高位隐睾引带组织;B:低位隐睾引带组织。红色信号示肌肉,可见典型横纹肌结构 Fig. 2 MY-32 immunofluorescent staining of gubernaculum testis from high/low cryptorchid children | |
高位组11例引带组织均未发现肌肉样结构,低位组62例中有43例引带组织可观察到横纹肌结构,差异有统计学意义(P < 0.001)。
讨 论人类隐睾睾丸位置的高低与睾丸发育程度及手术治疗效果密切相关,睾丸引带在胚胎期主要起牵引睾丸下降的作用[1, 6]。既往研究发现:人类胚胎睾丸在腹腔时睾丸引带组织内已存在横纹肌,提示人类睾丸引带的发育过程中含有肌肉成分,胚胎期睾丸引带内的横纹肌来源仍存在争议[7]。有学者认为是从腹壁肌肉组织延伸而来[8];也有学者认为是由引带内部的间充质干细胞分化而来[7]。已有研究在人类高低位隐睾的引带中发现其细胞外基质中总胶原纤维成分存在差异,但尚未有研究探究人类隐睾引带内肌肉组分的差异[5];因此,本研究旨在对高位和低位隐睾的引带进行肌肉组分的鉴定,并揭示其与高低位隐睾的关系。
本研究发现在高位隐睾患儿的引带中未见横纹肌,而低位组中可观察到横纹肌。人类睾丸下降的第一阶段为孕10~15周,睾丸从腹腔肾旁移行至内环口[9]。有文献报道在孕8周正常人类胚胎中可见引带内开始横纹肌演化,孕12周引带内已见典型横纹肌结构[7]。故在正常睾丸发育过程中,即使是处在高位阶段,引带内也应该存在横纹肌结构;而本研究发现高位隐睾患儿的引带中未观察到肌肉样组织,提示高位隐睾患儿其引带内横纹肌的发育可能存在异常。但因隐睾是一种出生后才可被明确诊断的以临床症状命名的疾病,在胚胎期尚无法推测其睾丸在出生后能否下降至正常位置,且人类胚胎睾丸引带采集涉及备受争议的伦理问题,故本研究采集的标本系隐睾形成后的引带而不是形成过程中的引带,所以尚无法直接判断是发育停滞还是引带本身发育不良引起的横纹肌缺乏。既往动物实验表明,腹腔内阶段睾丸的下降主要由睾丸Leydig细胞分泌的胰岛素样因子3(Insulin like 3、INSL3)调控。有文献研究证实INSL3基因敲除鼠及其受体RXFP2敲除鼠均为腹腔型隐睾表型,其引带表现为细长,且内部无肌肉组分[10, 11]。Ferlin等[12]提出INSL3与骨骼肌的分化存在联系,因其血清水平在人类不同生命阶段与人肌肉的质量呈特异性重叠趋势(在青春期后达到最大值,50岁后逐渐降低)[13]。以上研究均提示高位引带横纹肌的缺失可能与INSL3相关,具体机制仍需进一步研究。
人类睾丸下降第二阶段为经腹股沟阴囊移行期,发生在孕25~35周,此期在胚胎睾丸引带中可见横纹肌[14]。正常大鼠引带内横纹肌呈鞘状包绕,肌间隙呈纵向;而在隐睾大鼠模型中可观察到引带内肌肉存在反常的横向肌间隙、形状不对称、局部肌肉缺失及局灶性纤维不规则等异常特征[15]。在正常人类,该阶段其胚胎引带内肌肉也似鞘状包绕,但其肌纤维排列较啮齿类少且疏松[14, 16, 17];目前对人类低位隐睾引带内横纹肌的研究仅限于探究其含量,本研究通过组织切片观察人类低位隐睾引带中存在横纹肌结构,且通过骨骼肌特异性标记(MY-32)免疫荧光染色明确其为横纹肌[4]。我们观察到睾丸引带内肌肉组织大多为不规则排列,肌细胞分布较为分散,部分聚集呈束状;这提示低位隐睾的睾丸引带内横纹肌存在异常排列。Bouzada等[18]通过对19例人类早期胚胎,36例人类胎儿及8例新生儿进行引带及其周边器官的连续切片解剖观察,发现引带内可观察到横纹肌结构。而在本研究中低位组62例中19例未观察到横纹肌结构,究其原因可能为:①所采集的引带均为术中废弃引带,其完整度理论上较胚胎期整体取材低;②隐睾患儿的引带本身即是病理状态下发育的终点,其内横纹肌的缺失可能为引带发育异常所致。有研究报道雄激素参与了引带肌肉分化的过程,Kaftanovskaya等[19]通过Cre-LoxP基因编辑技术构建了组织特异性敲除引带内所有细胞(Rarb-Cre)的雄激素受体(AR)的隐睾模型小鼠,发现相对于正常小鼠其引带内Pax7(肌源干细胞标记)表达升高而ACTA1(骨骼肌标记)表达下降,提示雄激素/AR可能参与调节引带内横纹肌的发生。但Perer等[20]构建非组织特异性的AR敲除隐睾模型小鼠(其外生殖器表现型均为雌性),发现实验组的引带发育过程异常,但其引带的肌肉成熟相关标记(PAX7)与对照组比较无差异;考虑到全身敲除AR小鼠无法保持正常性征,其机体内分泌环境、引带周围微环境不如组织特异性敲除AR小鼠接近隐睾的病理生理状态,结合既往研究我们推测雄激素/AR途径是引带肌肉发生相关信号通路中的一部分,但其可能通过触发引带发育环境中的分子网络其他通路(如Wnt/β-catenin信号通路等)进而调节引带横纹肌的发生,其中具体机制有待进一步研究[21]。
本研究观察了人类高低位隐睾睾丸引带的组织学构成,发现在高位隐睾患儿的睾丸引带中未见横纹肌,而低位组中存在异常排列的横纹肌,但本文尚未对其发生的分子机制进一步挖掘,在本研究的基础上,未来可尝试通过动物实验胚胎期引带内肌肉发生的具体机制进行更深入的探究。
| 1 |
Favorito LA, Costa SF, Julio HR, et al. The importance of the gubernaculum in testicular migration during the human fetal period[J]. International Braz J Urol, 2014, 40(6): 722-729. DOI:10.1590/S1677-5538.Ibju.2014.06.02. |
| 2 |
Backhouse KM. The gubernaculum testis hunteri:testicular descent and maldescent. arris and gale lecture delivered at the royal college of surgeons of england on 27th october 1959[J]. Ann R Coll Surg Engl, 1964, 35: 15-33. |
| 3 |
Robbins AK, Mateson AB, Khandha A, et al. Fetal Rat Gubernaculum Mesenchymal Cells Adopt Myogenic and Myofibroblast-Like Phenotypes[J]. J Urol, 2016, 196(1): 270-278. DOI:10.1016/j.juro.2015.12.081. |
| 4 |
El Zoghbi CS, Favorito LA, Costa WS, et al. Structural analysis of gubernaculum testis in cryptorchid patients submitted to treatment with human chorionic gonadotrophin[J]. Int Braz J Urol, 2007, 33(2): 223-229. DOI:10.1590/S1677-55382007000200014. |
| 5 |
Soito IC, Favorito LA, Costa WS, et al. Extracellular matrix remodeling in the human gubernaculum during fetal testicular descent and in cryptorchidic children[J]. World J Urol, 2011, 29(4): 535-540. DOI:10.1007/s00345-011-0702-3. |
| 6 |
杨庆堂, 姚干, 梁健升, 等. 两种手术方式治疗儿童隐睾的比较研究[J]. 临床小儿外科杂志, 2018, 17(11): 862-865. DOI:10.3969/j.issn.1671-6353.2018.11.014. Yang QT, Yao G, Liang JS, et al. Clinical efficacies of different surgical approaches for cryptorchidism in children[J]. J Clin Ped Sur, 2018, 17(11): 862-865. DOI:10.3969/j.issn.1671-6353.2018.11.014. |
| 7 |
Tanyel FC, Talim B, Atilla P, et al. Myogenesis within the human gubernaculum:histological and immunohistochemical evaluation[J]. Eur J Pediatr Surg, 2005, 15(3): 175-179. DOI:10.1055/s-2005-837604. |
| 8 |
Van der Schoot P. Towards a rational terminology in the study of the gubernaculum testis:arguments in support of the notion that the cremasteric sac should be considered the gubernaculum in postnatal rats and other mammals[J]. J Anat, 1996, 189(Pt 1): 97-108. |
| 9 |
Hutson JM, Southwell BR, Li R, et al. The regulation of testicular descent and the effects of cryptorchidism[J]. Endocr Rev, 2013, 34(5): 725-752. DOI:10.1210/er.2012-1089. |
| 10 |
Tomiyama H, Hutson JM, Truong A, et al. Transabdominal testicular descent is disrupted in mice with deletion of insulinlike factor 3 receptor[J]. J Pediatr Surg, 2003, 38(12): 1793-1798. DOI:10.1016/j.jpedsurg.2003.08.047. |
| 11 |
Kaftanovskaya EM, Feng S, Huang Z, et al. Suppression of insulin-like3 receptor reveals the role of beta-catenin and Notch signaling in gubernaculum development[J]. Mol Endocrinol, 2011, 25(1): 170-183. DOI:10.1210/me.2010-0330. |
| 12 |
Ferlin A, De Toni L, Sandri M, et al. Relaxin and INSL3 in the muscolo-skeletal system:from bench to bedside[J]. Br J Pharmacol, 2017, 174(10): 1015-1024. DOI:10.1111/bph.13490. |
| 13 |
Bay K, Andersson AM. Human testicular insulin-like factor 3:in relation to development, reproductive hormones and andrological disorders[J]. Int J Androl, 2011, 34(2): 97-109. DOI:10.1111/j.1365-2605.2010.01074.x. |
| 14 |
Barteczko KJ, Jacob MI. The testicular descent in human.Origin, development and fate of the gubernaculum Hunteri, processus vaginalis peritonei, and gonadal ligaments[J]. Adv Anat Embryol Cell Biol, 2000, 156: Ⅲ-98. |
| 15 |
Barthold JS, Robbins A, Wang Y, et al. Cryptorchidism in the orl rat is associated with muscle patterning defects in the fetal gubernaculum and altered hormonal signaling[J]. Biol Reprod, 2014, 91(2): 41. DOI:10.1095/biolreprod.114.119560. |
| 16 |
Husmann DA, Levy JB. Current concepts in the pathophysiology of testicular undescent[J]. Urology, 1995, 46(2): 267-276. DOI:10.1016/S0090-4295(99)80207-6. |
| 17 |
Costa WS, Sampaio FJ, Favorito LA, et al. Testicular migration:remodeling of connective tissue and muscle cells in human gubernaculum testis[J]. J Urol, 2002, 167(5): 2171-2176. DOI:10.1016/S0022-5347(05)65122-1. |
| 18 |
Bouzada J, Vazquez T, Duran M, et al. New insights into the morphogenesis of the gubernaculum testis and the inguinal canal[J]. Clin Anat, 2017, 30(5): 599-607. DOI:10.1002/ca.22880. |
| 19 |
Kaftanovskaya EM, Huang Z, Barbara AM, et al. Cryptorchidism in mice with an androgen receptor ablation in gubernaculum testis[J]. Mol Endocrinol, 2012, 26(4): 598-607. DOI:10.1210/me.2011-1283. |
| 20 |
Perera N, Szarek M, Vannitamby A, et al. An immunohistochemical analysis of the effects of androgen receptor knock out on gubernacular differentiation in the mouse[J]. J Pediatr Surg, 2018, 53(9): 1776-1780. DOI:10.1016/j.jpedsurg.2017.11.063. |
| 21 |
Hughes IA, Acerini CL. Factors controlling testis descent[J]. Eur J Endocrinol, 2008, 159(Suppl 1): S75-S82. DOI:10.1530/eje-08-0458. |
 2019, Vol. 18
2019, Vol. 18


