2. 新疆医科大学第一附属医院小儿泌尿外科, 乌鲁木齐 830054
2. Department of Pediatric Urology, The First Affiliated Hospital of Xinjiang Medical University, Urumqi 830054, China
儿童肾脏恶性肿瘤约占所有儿童恶性肿瘤的6%,而威尔姆斯瘤(Wilms tumours,WT)约占儿童肾脏恶性肿瘤的90%[1]。WT通常是后肾胚胎分化停滞导致的结果,组织学上常表现为母细胞、基质细胞和上皮细胞的三相混合状态[2]。随着多学科协作的发展,WT患者的5年生存率已提高到90%左右,但仍存在预后较差的可能,因此WT的治疗趋势已转变为在不断提高现有生存率的同时,尽可能降低治疗强度,以降低治疗相关并发症的发生率,最终降低病死率[3-5]。
WT的发生和发展是一个复杂的生物学过程,包括微小非编码核糖核酸(microRNA, miRNA)、长链非编码核糖核酸(long non-coding RNA,lncRNA)、环状非编码核糖核酸(circularRNA, circRNA)等在内的非编码RNA均已被确定参与WT各阶段的调控[6]。其中miRNA是由18~25个核苷酸组成的非编码单链小RNA分子,在细胞凋亡、代谢、炎症和肿瘤发生等重要生物学过程中发挥重要的调控作用[7]。
核因子κB(nuclear factor kappa-B,NF-κB)信号通路持续激活可在miRNA的调控下促进炎症及WT的发生、发展,如miR-200b/c/429通过激活NF-κB通路促进WT的进展[8]。核因子κB抑制因子β(Nuclear factor kappa-B inhibitor beta,NFKBIB)属于核因子κB(Nuclear factor kappa-B,NF-κB)转录因子抑制蛋白家族,其磷酸化可激活NF-kB信号通路[9]。通过miRNA靶基因数据库TargetScan分析发现,NFKBIB可能存在miR-20a-5p的结合位点。既往研究表明miR-20a-5p通过调控HMGA2轴影响乳腺癌细胞生长、迁移、侵袭和凋亡,但miR-20a-5p对WT的影响及作用机制尚不明确[10-11]。因此本研究通过分析miR-20a-5p在293T及WIT49中的表达情况,探讨miR-20a-5p对人肾母细胞瘤增殖、迁移、侵袭及凋亡的影响及其作用机制。
资料与方法 一、实验材料 (一) 细胞系人胚肾细胞系293T及人肾母细胞瘤肺转移灶细胞系WIT49均购自武汉普诺赛生命科技有限公司。
(二) 主要试剂与耗材胎牛血清(fetal bovine serum,FBS)购自美国Gibco公司,miR-20a-5p过表达及干扰慢病毒载体购自上海吉凯基因科技有限公司,双荧光素酶报告系统试剂盒购自上海普洛麦格生物产品有限公司,细胞计数试剂盒购自北京博奥森生物技术有限公司,凋亡检测试剂盒购自比欧联科供应链管理(北京)有限公司,蛋白裂解液、二喹啉甲酸(bicinchoninic acid,BCA)蛋白定量试剂盒购自北京索莱宝科技有限公司,细胞RNA提取试剂盒购自康为世纪生物科技有限公司,逆转录和实时荧光定量聚合酶链反应试剂盒购自北京全式金生物技术有限公司,IKB-β抗体、p65抗体、内参(beta-actin,β-actin)抗体、山羊抗兔抗体、miR-20a-5p、NFKBIB引物购自上海生工生物工程有限公司。
二、实验方法 (一) 细胞培养人胚肾细胞系293T及人肾母细胞瘤肺转移灶细胞系WIT49用含10%胎牛血清的培养液培养,并在培养液中加入1%的青霉素和链霉素。在37℃、5% CO2培养箱中进行培养,待细胞密度达80% ~90%时,用1~2 mL 0.25% 胰蛋白酶消化液消化后传代。
(二) 慢病毒转染将细胞以2×105个/孔铺到6孔板中,分别标注miR-20a-5p过表达组、过表达对照组、miR-20a-5p低表达组、低表达对照组,待细胞密度达80% ~90%时,用含有6 μg/mL polybrene的2 mL新鲜培养基替换原培养基,加入病毒悬液,37℃孵育。病毒悬液miR-20a-5p过表达组滴度:1×106 TU/mL;过表达及低表达对照组滴度:1×107 TU/mL;miR-20a-5p低表达组滴度:1×106 TU/mL。在37℃、5% CO2培养箱中进行培养,显微镜观察荧光蛋白,转染效率达80%时用6 μg/mL嘌呤霉素筛选稳度表达细胞系。
(三) 双荧光素酶酶实验靶基因预测软件TargetScan预测结果显示,NFKBIB与miR-20a-5p具有结合位点(h-NFKBIB-3UTR-wt: 5'-ACGCCUGUAAUCCCAGCACUUUG-3', hsa-miR-20a-5p: 3'-GAUGGACGUGAUAUUCGUGAAAU-5')。通过双荧光素酶实验验证,将用于转染的293T细胞放入96孔板中,待细胞密度达50% ~70%时,将培养基与目的质粒、目标基因充分混匀后室温放置(溶液A),之后将培养基与转染试剂充分混匀放置(溶液B),将溶液A与溶液B充分混匀,室温放置20 min,将转染混合物加入96孔板混匀,37℃、5% CO2培养箱中进行培养48 h后收集细胞,使用双荧光素酶报告系统进行检测。
(四) 总RNA提取和qRT-PCR检测采用Trizol法提取总RNA,检测其浓度,取5 000 ng RNA样品逆转录得到cDNA。取2.0 μL cDNA为模版,对miR-20a-5p及NFKBIB进行qRT-PCR扩增检测,内参为β-actin。PCR反应体系为20 μL,按照qRT-PCR试剂盒说明书操作(PCR运行条件:94℃ 30 s,94℃ 5 s,55℃ 30 s,40个循环)。采用2-ΔΔCt法计算各组细胞中miR-20a-5p及NFKBIB的相对表达量。
(五) CCK-8实验将转染成功的WIT49细胞制备细胞悬液并计数后接种在96孔细胞培养板中,每孔加入1×105个细胞/mL细胞悬液100 μL,37℃、5% CO2培养箱培养0 h、24 h、48 h、72 h后,避光下每孔加10 μL CCK-8试剂,继续培养2 h后用酶标仪测定其在450 nm条件下的吸光度。
(六) 划痕实验将转染后的WIT49细胞接种在6孔板中,密度达90%左右时弃上清液,用无菌枪头在6孔板底部垂直划出3条直线,PBS清洗2次,将脱落细胞清洗干净,加入无血清培养基继续培养。在显微镜下分别观察划痕0~24 h细胞愈合面积。
(七) Transwell侵袭、迁移实验将转染后的WIT49细胞制备悬液并计数后接种到含有基质胶的Transwell小室,每孔加入1×106个细胞/mL细胞悬液200 μL,下层加入含有15%血清的细胞培养基500 μL,放入含5% CO2的37℃培养箱中培养48 h后取出Transwell小室用PBS清洗2次,用4%的多聚甲醛固定10 min。加入0.1%结晶紫染色液染色,显微镜下观察、拍照并计算穿透微孔膜的细胞数量。
(八) 流式凋亡实验用不含乙二胺四乙酸的胰蛋白酶消化液消化转染后的WIT49细胞制成细胞悬液,收集上清液并离心,PBS清洗2次后加入1×binding buffer重悬细胞并将其移到流式管中,根据试剂盒说明书要求加入工作液,室温条件下避光孵育15 min,上机分析。
(九) 蛋白质印迹法(Western blot)按照蛋白酶抑制剂与裂解液1 ∶ 10提取细胞蛋白质,采用BCA法测定蛋白浓度。凝胶制备后,加入6 μg预染蛋白Marker,20 μg蛋白样品,80 V电压电泳30 min后改为100 V电压继续电泳90 min。电泳后的凝胶用100 V电压电转120 min至硝酸纤维素膜上。PTST漂洗后,浸入脱脂牛奶封闭1 h 50 min,加入IKB-β、p65一抗、内参β-actin过夜。TBST清洗后,浸入抗兔IgG二抗温孵育50 min,再用TBST清洗3次后显影检测。
三、统计学处理采用SPSS 26.0进行统计学分析,并使用GraphPad Prism 8绘制统计图。服从正态分布的计量资料用x±s表示,两组间比较采用两独立样本t检验;三组及以上组间比较采用单因素方差分析,整体差异有统计学意义的基础上,两两比较采用LSD-t检验。P < 0.05为差异具有统计学意义。
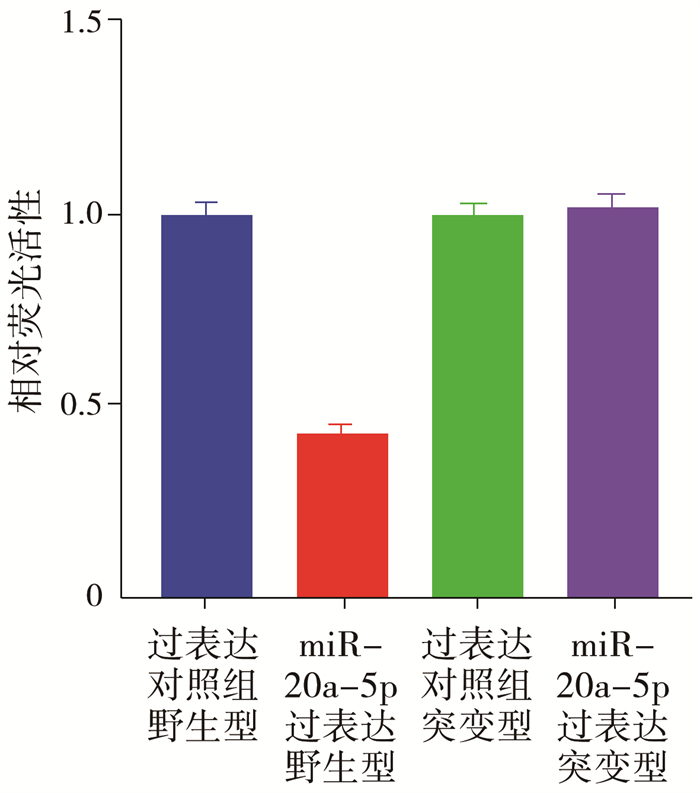
结果 一、双荧光素酶结果显示miR-20a-5p可抑制NFKBIB的表达miR-20a-5p过表达组与过表达对照组的野生型荧光素酶活性比较,差异有统计学意义(P<0.01);miR-20a-5p过表达组与过表达对照组的突变型荧光素酶活性比较,差异无统计学意义(P>0.05)。见图 1。
|
图 1 荧光素酶报告实验检测miR-20a-5p与NFKBIB作用的关系 Fig.1 Results of the luciferase reporter assay detecting the relationship between miR-20a-5p and NFKBIB |
WIT49中miR-20a-5p表达显著高于293T[(1.97±0.79)比(1.01±0.20)],NFKBIB表达低于293T[(0.26±0.08)比(1.02±0.26)];miR-20a-5p过表达组miR-20a-5p表达高于过表达对照组[(2.30±0.97)比(1.02±0.23)],miR-20a-5p低表达组miR-20a-5p表达低于低表达对照组[(0.45±0.14)比(1.01±0.15)];miR-20a-5p过表达组NFKBIB表达低于过表达对照组[(0.53±0.20)比(1.01±0.17)],miR-20a-5p低表达组NFKBIB表达高于低表达对照组[(2.47±1.06)比(1.00±0.09)];上述指标差异均具有统计学意义(P<0.01)。
三、CCK-8实验结果显示miR-20a-5p可促进WIT49细胞增殖0 h转染各组WIT49细胞光密度值差异无统计学意义(P>0.05);miR-20a-5p过表达组24 h、48 h细胞光密度值为(0.36±0.03)、(1.76±0.03), 均高于过表达对照组24 h、48 h细胞光密度值(0.18±0.01)、(0.87±0.04),差异均具有统计学意义(P<0.01);miR-20a-5p过表达组、过表达对照组72 h细胞光密度值分别为(2.83±0.05)、(2.84±0.04),差异不具有统计学意义(P=0.90);miR-20a-5p低表达组24 h、48 h、72 h细胞光密度值为(0.03±0.05)、(0.61±0.01)、(1.80±0.03),均低于低表达对照组24 h、48 h、72 h细胞光密度值(0.11±0.01)、(0.85±0.01)、(2.72±0.06),差异均具有统计学意义(P<0.01)。
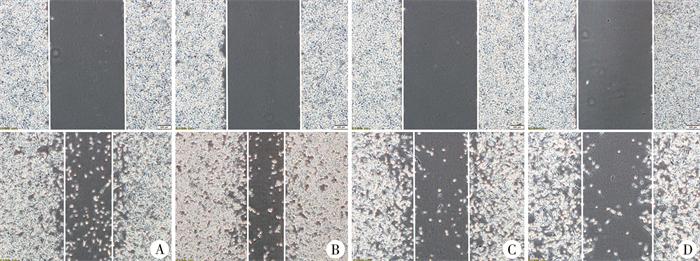
四、划痕实验结果显示miR-20a-5p可促进WIT49细胞迁移miR-20a-5p过表达组24 h划痕愈合率高于过表达对照组[(53.985±0.275)%比(34.87±0.08)%],见图 2A、2B;miR-20a-5p低表达组24 h划痕愈合率低于低表达对照组[(16.56±0.09)%比(34.74±0.15)%],差异均具有统计学意义(P<0.01),见图 2C、2D。
|
图 2 划痕实验检测转染后各组WIT49细胞迁移能力(bar=200 μm) Fig.2 Scratch assay detecting migration ability of transfected WIT49 cell groups (bar=200 μm) 注 A:过表达对照组24 h愈合面积;B:miR-20a-5p过表达组24 h愈合面积;C:低表达对照组24 h愈合面积;D:miR-20a-5p低表达组24 h愈合面积 |
miR-20a-5p过表达组穿透微孔膜的细胞个数高于过表达对照组[(88.67±11.20)比(51.00±7.45)],见图 3A、3B;miR-20a-5p低表达组穿透微孔膜的细胞个数低于低表达对照组[(23.67±7.59)比(53.67±10.35)],差异均具有统计学意义(P<0.01), 见图 3C、3D。

|
图 3 Transwell迁移实验检测转染后各组WIT49细胞迁移能力(Bar=100 μm) Fig.3 Transwell migration assay was used to detect the migration ability of WIT49 cells after transfection(Bar=100 μm) 注 A:miR-20a-5p过表达组24 h穿透微孔膜的细胞个数;B:过表达对照组24 h穿透微孔膜的细胞个数;C:miR-20a-5p低表达组24 h穿透微孔膜的细胞个数;D:低表达对照组24 h穿透微孔膜的细胞个数 |
Transwell侵袭实验中miR-20a-5p过表达组穿透微孔膜的细胞个数高于过表达对照组[(64.00±18.75)比(24.33±7.59)],见图 4A、4B;miR-20a-5p低表达组穿透微孔膜的细胞个数低于低表达对照组[(3.33±1.44)比(25.00±4.30)],差异具有统计学意义(P<0.01),见图 4C、4D。

|
图 4 Transwell侵袭实验检测转染后各组WIT49细胞侵袭能力(Bar=100 μm) Fig.4 Transwell invasion assay was used to detect the invasion ability of WIT49 cells in each group after transfection (Bar=100 μm) 注 A:miR-20a-5p过表达组48 h穿透微孔膜的细胞个数;B:过表达对照组48 h穿透微孔膜的细胞个数;C:miR-20a-5p低表达组48 h穿透微孔膜的细胞个数;D:低表达对照组48 h穿透微孔膜的细胞个数 |
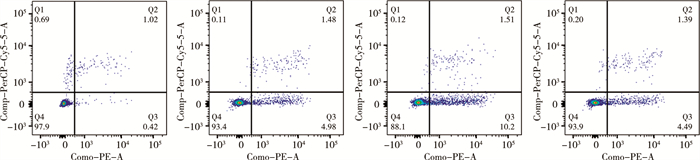
流式细胞凋亡实验结果显示miR-20a-5p过表达组凋亡细胞比例低于过表达对照组[(1.87±0.89)%比(6.42±0.48)%]; miR-20a-5p低表达组凋亡细胞比例高于低表达对照组[(11.33±0.91)%比(6.07±0.58)%];上述指标差异均具有统计学意义(P<0.01),见图 5。
|
图 5 流式凋亡实验检测转染后各组WIT49细胞的凋亡水平 Fig.5 Flow apoptosis assay to detect the apoptosis level of WIT49 cells in each group after transfection. 注 A:miR-20a-5p过表达组流式凋亡实验结果;B:过表达对照组流式凋亡实验结果;C:miR-20a-5p低表达组流式凋亡实验结果;D:低表达对照组流式凋亡实验结果 |
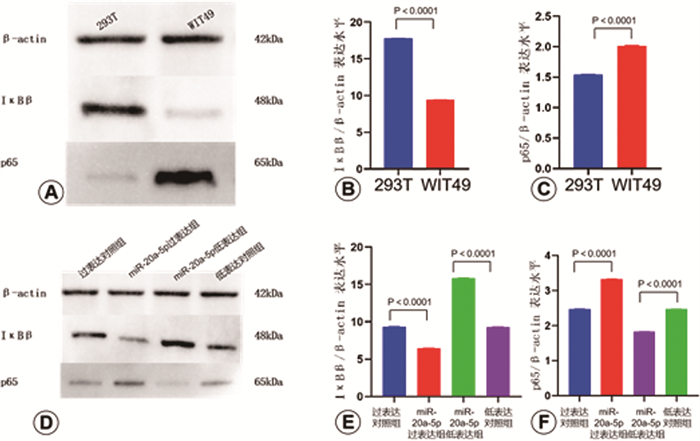
293T中IκBβ表达高于WIT49[(17.81±0.08)比(9.42±0.05)],p65表达低于WIT49[(1.54±0.01)比(2.02±0.02)];miR-20a-5p过表达组IκBβ表达低于过表达对照组[(6.45±0.01)比(9.35±0.02)],miR-20a-5p低表达组IκBβ表达高于低表达对照组[(15.87±0.04)比(9.32±0.05)],miR-20a-5p过表达组p65表达高于过表达对照组[(3.33±0.01)比(2.46±0.03)],miR-20a-5p低表达组p65表达低于低表达对照组[(1.82±0.02)比(2.46±0.02)]; 上述指标差异均具有统计学意义(P<0.01),见图 6。
|
图 6 Western blot检测293T、WIT49及转染后各组细胞中IκBβ及p65蛋白表达水平 Fig.6 Western blot detecting IκBβ and p65 protein expression in 293T, WIT49, and transfected cell groups 注 A:Western blot检测293T及WIT49细胞中内参β-actin、IκBβ及p65表达水平;B:Western blot检测293T中IκBβ蛋白表达水平高于WIT49细胞;C:Western blot检测293T中p65蛋白表达水平低于WIT49细胞;D:Western blot检测转染后各组WIT49细胞中β-actin、IκBβ及p65表达水平;E:Western blot检测WIT49细胞中miR-20a-5p抑制IκBβ表达;F:Western blot检测WIT49细胞中miR-20a-5p促进p65表达 |
本研究结果提示NFKBIB是miR-20a-5p直接靶基因,miR-20a-5p可能是通过调控NFKBIB激活NF-κB通路促进人肾母细胞瘤增殖、迁移、侵袭,抑制其凋亡。儿童肿瘤学组(Children's Oncology Group,COG)和国际儿科肿瘤学会(International Society of Pediatric Oncology,SIOP)开展了一项大型多中心研究,确定了WT患者当前的治疗方案,COG提倡先进行WT的原发灶切除,然后根据患者自身情况进行辅助治疗;SIOP提倡先行新辅助化疗,然后切除病灶,术后再行辅助治疗。两种方案均使得WT患儿的总生存率得到了显著提升[12]。然而,不同病理类型患儿预后具有显著差异,部分患儿治疗后出现了复发或严重晚期并发症,因此针对WT的研究目标已转变为优化风险分级以及治疗策略,而生物学标志物的探索使风险导向治疗成为可能[5, 13]。
miRNA可调控超过60%的人类蛋白编码基因,并调节包括肾脏发育在内的多种生物过程,miRNA表达失调会破坏早期肾脏发育,并与发育性肾脏疾病(如先天性肾脏、尿路畸形及WT)的发病机制有关[14]。此前miRNA已作为潜在的新型生物标志物在WT中被广泛研究[15]。如miR-21过表达可以通过负调控磷酸酶和张力蛋白磷酸酶同源物(phosphatase and tensin homolog,PTEN)促进WT细胞侵袭功能[16]。此外,同一miRNA在一种癌症中可能被视为肿瘤促进因子,而在另一种癌症中可能被视为肿瘤抑制因子[14]。如miR-20a-5p在宫颈癌、乳腺癌和白血病中具有抑癌作用,而在膀胱癌中miR-20a-5p通过负调节核受体4A3(nuclear receptor 4A3,NR4A3)促进癌症转移,与本研究miR-20a-5p促进WT细胞功能具有一致性[17]。
NF-κB家族是一组在诱导炎症及肿瘤发生、发展反应中起关键作用的转录因子,NF-κB抑制蛋白IκBα、IκBβ和IκBε通过在细胞质中隔离NF-κB亚基的复合体来调节NF-κB转录[9]。当IκB蛋白被IκB激酶复合物磷酸化时,NF-κB蛋白释放并定位于细胞核,促进炎症及肿瘤的发展。研究表明NF-κB在WT发生、发展过程中发挥核心作用,如NF-κB可以调控WT的抑癌基因WT1影响WT的发生,邻苯二甲酸单乙基己酯(monoethylhexyl phthalate, MEHP)通过激活NF-κB促进WT的迁移和侵袭。
本研究检测了可能与NFKBIB具有相互作用位点的miR-20a-5p在293T及WIT49中的表达情况,探究了miR-20a-5p对人肾母细胞瘤细胞功能的影响及作用机制,发现miR-20a-5p可能是通过调控NFKBIB激活NF-κB通路促进人肾母细胞瘤增殖、迁移、侵袭,抑制其凋亡。
利益冲突 所有作者声明不存在利益冲突
作者贡献声明 文献检索为王雅琦、李万富; 实验过程为王雅琦、李万富、阿依古再丽·麦麦江、艾尼娃·克然木; 数据分析为王雅琦、刘辉; 论文结果撰写为王雅琦、樊珈榕; 论文讨论分析为王雅琦、帕洛克·迪力木拉提
| [1] |
Steliarova-Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001-10:a population-based registry study[J]. Lancet Oncol, 2017, 18(6): 719-731. DOI:10.1016/S1470-2045(17)30186-9 |
| [2] |
Behjati S, Gilbertson RJ, Pfister SM. Maturation block in childhood cancer[J]. Cancer Discov, 2021, 11(3): 542-544. DOI:10.1158/2159-8290.CD-20-0926 |
| [3] |
Brok J, Mavinkurve-Groothuis AMC, Drost J, et al. Unmet needs for relapsed or refractory Wilms tumour: Mapping the molecular features, exploring organoids and designing early phase trials-A collaborative SIOP-RTSG, COG and ITCC session at the first SIOPE meeting[J]. Eur J Cancer, 2021, 144: 113-122. DOI:10.1016/j.ejca.2020.11.012 |
| [4] |
van den Heuvel-Eibrink MM, Hol JA, Pritchard-Jones K, et al. Rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol[J]. Nat Rev Urol, 2017, 14(12): 743-752. DOI:10.1038/nrurol.2017.163 |
| [5] |
方一圩, 宋宏程. 儿童不同病理类型肾脏肿瘤的鉴别与诊断[J]. 临床小儿外科杂志, 2022, 21(12): 1106-1110. Fang YW, Song HC. Differential diagnosis of different pathologic types of renal tumors in children[J]. Journal of Clinical Pediatric Surgery, 2022, 21(12): 1106-1110. DOI:10.3760/cma.j.cn101785-202205056-002 |
| [6] |
de Sá Pereira BM, Montalvão de Azevedo R, da Silva Guerra JV, et al. Non-coding RNAs in Wilms'tumor: biological function, mechanism, and clinical implications[J]. J Mol Med (Berl), 2021, 99(8): 1043-1055. DOI:10.1007/s00109-021-02075-1 |
| [7] |
Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function[J]. Nucleic Acids Res, 2019, 47(D1): D155-D162. DOI:10.1093/nar/gky1141 |
| [8] |
Liu P, Chen SF, Huang YY, et al. LINC00667 promotes Wilms'tumor metastasis and stemness by sponging miR-200b/c/429 family to regulate IKK-β[J]. Cell Biol Int, 2020, 44(6): 1382-1393. DOI:10.1002/cbin.11334 |
| [9] |
Karin M. Nuclear factor-kappaB in cancer development and progression[J]. Nature, 2006, 441(7092): 431-436. DOI:10.1038/nature04870 |
| [10] |
Zhao WY, Geng DH, Li SQ, et al. LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer[J]. Cancer Med, 2018, 7(3): 842-855. DOI:10.1002/cam4.1353 |
| [11] |
Nakata KY, Colombet M, Stiller CA, et al. Incidence of childhood renal tumours: an international population-based study[J]. Int J Cancer, 2020, 147(12): 3313-3327. DOI:10.1002/ijc.33147 |
| [12] |
Dome JS, Graf N, Geller JI, et al. Advances in Wilms tumor treatment and biology: progress through international collaboration[J]. J Clin Oncol, 2015, 33(27): 2999-3007. DOI:10.1200/JCO.2015.62.1888 |
| [13] |
Spreafico F, Fernandez CV, Brok J, et al. Wilms tumour[J]. Nat Rev Dis Primers, 2021, 7(1): 75. DOI:10.1038/s41572-021-00308-8 |
| [14] |
Smith CM, Catchpoole D, Hutvagner G. Non-coding RNAs in pediatric solid tumors[J]. Front Genet, 2019, 10: 798. DOI:10.3389/fgene.2019.00798 |
| [15] |
Roberti A, Valdes AF, Torrecillas R, et al. Epigenetics in cancer therapy and nanomedicine[J]. Clin Epigenetics, 2019, 11(1): 81. DOI:10.1186/s13148-019-0675-4 |
| [16] |
Cui MY, Liu W, Zhang LJ, et al. Over-expression of miR-21 and lower PTEN levels in Wilms'tumor with aggressive behavior[J]. Tohoku J Exp Med, 2017, 242(1): 43-52. DOI:10.1620/tjem.242.43 |
| [17] |
Huang W, Wu XY, Xiang SX, et al. Regulatory mechanism of miR-20a-5p expression in Cancer[J]. Cell Death Discov, 2022, 8(1): 262. DOI:10.1038/s41420-022-01005-5 |
 2024, Vol. 23
2024, Vol. 23


