发育性髋关节脱位临床表现为髋臼发育不良、髋关节全脱位或半脱位,是儿童常见的骨关节发育疾病。闭合复位是临床治疗该病的常用方法,旨在保证早期稳定复位,降低股骨头缺血性坏死(avascular necrosis of femoral head, AVN)的风险[1-2]。AVN是发育性髋关节脱位闭合复位治疗后常见的并发症,既往研究显示,AVN的发生与脱位程度、制动体位、石膏固定方式、治疗年龄等因素有关,但与AVN发生相关的实验室指标较少[3-4]。骨桥蛋白(osteopontin, OPN)具有诱导骨骼钙化、免疫反应、细胞黏附和迁移等多种生物学功能,在血管重塑、损伤修复和抗炎等方面均有一定作用[5-6]。瘦素是一种分泌型蛋白质,能够引起脂肪代谢紊乱,而脂肪代谢紊乱是股骨头坏死的重要机制[7]。本研究回顾性分析130例发育性髋关节脱位患儿的临床资料,旨在探究OPN、瘦素联合临床特征预测发育性髋关节脱位并发AVN的价值,以期为临床筛查AVN高风险人群提供理论依据,并为临床采取干预性措施提供指导。
资料与方法 一、研究对象回顾性分析2018年1月至2021年1月海南省妇女儿童医学中心收治的130例发育性髋关节脱位患儿临床资料。病例纳入标准:①单侧髋关节脱位;②影像学资料完整;③行闭合复位治疗;④患儿父母对本研究知情同意。排除标准:①感染性髋关节脱位;②合并骨髓病变(如脊髓发育异常、脊髓肿块等);③脑瘫性髋关节脱位;④合并脊髓栓系、脑瘫、马蹄内翻足等疾病;⑤合并骨代谢疾病(如骨质疏松、内分泌骨病等)。研究已获得海南省妇女儿童医学中心伦理委员会批准(2017-23号)。纳入研究患儿中男59例、女71例;年龄(1.78±0.42)岁;左髋63例、右髋67例;血管分型:Ⅰ型20例、Ⅱ型46例、Ⅲ型64例;股骨头灌注改变:A类57例、B类56例、C类17例。患儿于全身麻醉下行闭合复位治疗,复位后石膏固定3个月,拆除石膏后予全天外展支具固定3个月,随访18个月,末次随访时对所有患儿行X线检查,采用Kalamchi-MacEwen法评估AVN情况,根据是否发生AVN分为AVN组(n=52)和无AVN组(n=78)。
二、研究方法收集所有患儿临床资料,包括性别、年龄、患髋侧别、脱位程度、内收肌松解情况、有无骨化核、血管分型、血管数量、术前髋臼指数、术后股骨头灌注改变情况。
股骨头测量:在影像归档和通信系统(picture archiving and communication system, PACS)中,采集患儿闭合复位治疗前MRI检查冠状面、横断面股骨头最大扫描层并测量以下指标:①股骨头高度:划定股骨近端骺板线,之后平行此线在股骨头顶端作一条切线,股骨头高度即为两条线之间的距离;②股骨头左右径:在股骨头内外侧各作一条切线,并保证两条线都与股骨头近端骺板线垂直,股骨头直径即为两条切线间的距离;③股骨头前后径:在股骨头前后侧各作一条水平切线,股骨头前后径即为两条水平切线的距离。
实验室检测指标:闭合复位治疗前采集患儿空腹外周静脉血4 mL,以3 000 r/min离心10 min,取血清置于-80℃冰箱中保存待检。严格遵循试剂盒步骤,采用酶联免疫吸附法检测血清OPN、瘦素水平。
三、统计学处理采用SPSS 22.0对数据进行处理。服从正态分布的计量资料(如年龄、血管数量等)以x±s表示,组间比较采用两独立样本t检验;计数资料(如性别、患髋侧别)以例(%)表示,组间比较采用χ2检验;多因素分析采用非条件Logistic逐步回归分析;以受试者工作特征(receiver operating characteristic,ROC)曲线分析股骨头左右径、股骨头前后径、血清OPN及瘦素预测发育性髋关节脱位患儿并发AVN的价值。P<0.05为差异有统计学意义。
结果 一、发育性髋关节脱位患儿并发AVN的单因素分析与无AVN组相比,AVN组血管分型Ⅰ型、术后股骨头灌注改变为B类和C类的患者比例较高,且AVN组股骨头左右径、股骨头前后径较小,血清OPN、瘦素水平较高(P<0.05);两组性别、年龄、患髋侧别、脱位程度、内收肌松解情况、有无骨化核、血管数量、术前髋臼指数、股骨头高度等资料比较差异无统计学意义(P>0.05),见表 1。
| 表 1 影响发育性髋关节脱位患儿并发AVN的单因素分析 Table 1 Univariate analysis of femoral head necrosis in DHD children |
|
|
将单因素分析中有统计学意义的变量纳入Logistic多因素回归模型,进行判定量化处理,因变量为发育性髋关节脱位患儿是否并发AVN(是=1,否=0),自变量为血管分型(Ⅰ型=1,Ⅱ、Ⅲ型=0)、术后股骨头灌注改变类别(B、C类=1,A类=0)、股骨头左右径(≤1.782 cm=1,>1.782 cm=0)、股骨头前后径(≤1.856 cm=1,>1.856 cm=0)、OPN(≥8.049 μg/L=1,<8.049 μg/L=0)、瘦素(≥38.075 μg/L=1,<38.075 μg/L=0)。结果显示,血管分型Ⅰ型、术后股骨头灌注改变为B类和C类、股骨头左右径≤1.782 cm、股骨头前后径≤1.856 cm、OPN≥8.049 μg/L、瘦素≥38.075 μg/L是发育性髋关节脱位患儿并发AVN的危险因素(P<0.05),见表 2。
| 表 2 影响发育性髋关节脱位患儿并发AVN的多因素分析结果 Table 2 Multivariate Logistic regression analysis of femoral head necrosis in DHD children |
|
|
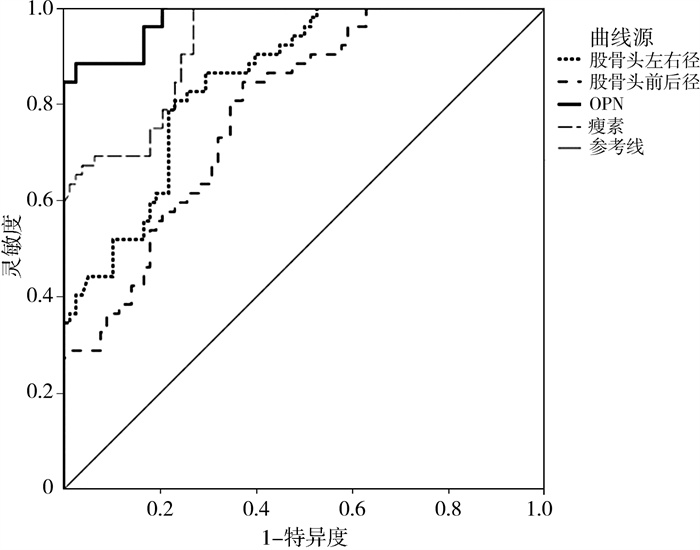
经ROC曲线分析发现,股骨头左右径≤1.782 cm、股骨头前后径≤1.856 cm、OPN≥8.049 μg/L、瘦素≥38.075 μg/L是发育性髋关节脱位患儿并发AVN的最佳截断值(P<0.05),见表 3;股骨头左右径、股骨头前后径、血清OPN及瘦素预测发育性髋关节脱位患儿并发AVN的ROC曲线见图 1。
| 表 3 股骨头左右径、股骨头前后径、血清OPN及瘦素预测发育性髋关节脱位患儿并发AVN的ROC曲线分析 Table 3 ROC analysis of left/right femoral head diameter, anterioposterior femoral head diameter and serum OPN/leptin in predicting femoral head necrosis in DHD children |
|
|
|
图 1 股骨头左右径、股骨头前后径、血清OPN及瘦素预测发育性髋关节脱位患儿并发AVN的ROC曲线 Fig.1 ROC curve of left/right femoral head diameter, anterioposterior femoral head diameter and serum OPN/leptin in predicting femoral head necrosis in DHD children 注 AVN:股骨头缺血性坏死;OPN:骨桥蛋白;ROC:受试者工作特征 |
AVN是发育性髋关节脱位闭合复位治疗后常见且较严重的并发症,发生AVN后,患儿会遗留不同程度的股骨头畸形,包括肢体不等长、短髋畸形、股骨头变形等,最后可导致退行性关节炎,引起严重的功能障碍和疼痛,对患儿身心健康造成严重影响[8-10]。AVN的发病机制和预防是临床研究的重要课题,目前国内外对AVN发生的影响因素研究较多,但结论尚未统一,且缺乏可以量化的指标。本研究通过分析OPN、瘦素联合临床特征预测发育性髋关节脱位患儿并发AVN的价值,期望为AVN的预防提供帮助。
本研究发现,与无AVN组相比,AVN组血管分型为Ⅰ型、术后股骨头灌注改变为B类和C类的患者比例较高,且AVN组股骨头左右径及前后径较小,血清OPN、瘦素水平较高,提示以上因素可能是发育性髋关节脱位并发AVN的相关因素,考虑其原因为: ①股骨头的正常发育和骨化与其血供密切相关,随着股骨头骨骼的生长发育,股骨头的血管数量和分布也不断改变[11]。研究显示,婴幼儿的正常股骨头血管主要呈现出三种类型,并与骨骺发育Ⅰ、Ⅱ、Ⅲ型相对应,分型越高,血管发育越成熟[12]。本研究中AVN组血管分型为Ⅰ型的患儿比例明显高于无AVN组,即AVN组血管发育不成熟的患儿较多,股骨头发育较差,因此发生AVN的风险较高。②本研究中AVN组股骨头灌注改变为B、C类的患儿比例较高,提示患儿闭合复位后血流灌注变差,血运不佳,既往研究已证实导致AVN发生的一个重要因素为股骨头血运障碍[13]。③股骨头发育情况与AVN的发生具有密切联系,研究显示,随着股骨头直径的增加,AVN的发生率明显降低,其抗压面积也越大,循环压应力小;另外随着股骨头直径的增多,血管数量相应增多,血管管腔也变大,因此股骨头直径与AVN的发生风险呈负相关[14-15]。④OPN对AVN的影响可能与以下因素有关:其一,OPN能通过减少组织中蛋白多糖、提高基质金属蛋白酶13等方式,促进软骨退行性变,且OPN可以诱导软骨细胞凋亡,从而对关节软骨造成损害[16]。其二,OPN在骨吸收时能促进骨基质和破骨细胞的黏附,且OPN还可诱导破骨细胞的破骨过程。有研究认为,骨桥蛋白能够引起骨代谢改变,具有介导机械应力的作用[17]。⑤瘦素对AVN的影响可能与以下因素有关:第一,瘦素具有调节骨量的作用,瘦素能够通过介导患者体内脂质和骨量的平衡,在骨代谢、能量平衡、储备体脂等过程中发挥纽带作用[18]。第二,瘦素具有调控骨形成的作用,瘦素在中枢神经系统中是骨形成的抑制因子[19]。第三,瘦素在骨矿化、成骨细胞发育过程中具有重要作用,研究显示,在体外培养的成骨细胞中加入瘦素能够刺激并促进细胞增殖、分化和矿化,此外瘦素还可通过减少细胞凋亡促进成骨细胞向骨细胞转化[20]。
本研究使用Logistic回归分析模型及ROC曲线进行分析,结果显示血管分型Ⅰ型、股骨头灌注改变为B和C类、股骨头左右径≤1.782 cm、股骨头前后径≤1.856 cm、OPN≥8.049 μg/L、瘦素≥38.075 μg/L是发育性髋关节脱位患儿并发AVN的危险因素,可以认为符合以上因素的患儿发生AVN的风险较高,临床应密切关注。
综上,股骨头血管发育不成熟、闭合复位术后股骨头灌注异常、股骨头直径等因素可能增加发育性髋关节脱位患儿发生AVN的风险。临床可通过测量股骨头直径,检测血清OPN、瘦素水平筛查AVN高风险患儿,及时干预,降低AVN发生率。此外本研究仍存在部分不足之处,如样本量较小、存在一定偏倚、未能将AVN的所有影响因素纳入分析等,希望在后续研究中扩大样本量,进一步明确临床特征与AVN发生之间的关系。
利益冲突 所有作者声明不存在利益冲突
作者贡献声明 黄乙勇负责研究的设计、实施和起草文章;符凯进行病例数据收集及分析;洪聪、朱立宁负责研究设计与酝酿,并对文章知识性内容进行审阅
| [1] |
孙庆增, 沈阳, 戚玉东, 等. 经内侧与前侧入路切开复位治疗发育性髋关节脱位临床疗效分析[J]. 中华实用诊断与治疗杂志, 2021, 35(3): 251-254. Sun QZ, Shen Y, Qi YD, et al. Clinical outcomes of open reduction via medial and anterior approaches for developmental dysplasia of the hip[J]. J Chin Pract Diagn Ther, 2021, 35(3): 251-254. DOI:10.13507/j.issn.1674-3474.2021.03.010 |
| [2] |
杜敏东, 刘雄, 秦刚, 等. 髋关节外科脱位头颈开窗打压植骨术联合活血补肾方治疗中晚期肾虚血瘀型股骨头坏死的临床疗效[J]. 广西医学, 2019, 41(22): 2821-2824. Du MD, Liu X, Qin G, et al. Clinical efficacy of impaction bone-grafting after surgical hip dislocation and head and neck fenestration plus Huoxue Bushen recipe for middle/late-stage osteonecrosis of the femoral head with kidney deficiency and blood stasis[J]. Guangxi Med J, 2019, 41(22): 2821-2824. DOI:10.11675/j.issn.0253-4304.2019.22.01 |
| [3] |
Li YQ, Zhou QH, Liu YZ, et al. Closed reduction and dynamic cast immobilization in patients with developmental dysplasia of the hip between 6 and 24 months of age[J]. Eur J Orthop Surg Traumatol, 2019, 29(1): 51-57. DOI:10.1007/s00590-018-2289-5 |
| [4] |
Sankar WN, Gornitzky AL, Clarke NMP, et al. Closed reduction for developmental dysplasia of the hip: early-term results from a prospective, multicenter cohort[J]. J Pediatr Orthop, 2019, 39(3): 111-118. DOI:10.1097/BPO.0000000000000895 |
| [5] |
于彩霞, 巩云霏, 闫苏, 等. 血清视黄醇结合蛋白4及骨桥蛋白与绝经后骨质疏松症的相关性研究[J]. 现代检验医学杂志, 2020, 35(2): 112-115, 118. Yu CX, Gong YF, Yan S, et al. Relationship between serum retinol binding protein 4 and osteopontin in postmenopausal osteoporosis[J]. J Mod Lab Med, 2020, 35(2): 112-115, 118. DOI:10.3969/j.issn.1671-7414.2020.02.031 |
| [6] |
王健, 陈瑶, 黄培基, 等. 绝经后妇女骨密度和血清骨转换指标与骨桥蛋白水平的相关性研究[J]. 中国骨质疏松杂志, 2019, 25(1): 23-28. Wang J, Chen Y, Huang PJ, et al. Relationship among bone mineral density, serum bone turnover parameters and osteopontin in postmenopausal women[J]. Chin J Osteoporos, 2019, 25(1): 23-28. DOI:10.3969/j.issn.1006-7108.2019.01.005 |
| [7] |
梁杰, 崔蕴文, 赵樱. 血清瘦素、脂联素及趋化素水平与老年人群骨质疏松相关性研究[J]. 实用老年医学, 2022, 36(2): 184-187. Liang J, Cui YW, Zhao Y. Correlation between osteoporosis and serum levels of leptin, adiponectin and chemokine in elders[J]. Pract Geriatr, 2022, 36(2): 184-187. DOI:10.3969/j.issn.1003-9198.2022.02.019 |
| [8] |
Li YQ, Guo YM, Shen XT, et al. Radiographic outcome of children older than twenty-four months with developmental dysplasia of the hip treated by closed reduction and spica cast immobilization in human position: a review of fifty-one hips[J]. Int Orthop, 2019, 43(6): 1405-1411. DOI:10.1007/s00264-019-04315-z |
| [9] |
Milenkovic S, Mitkovic M, Mitkovic M. Avascular necrosis of the femoral head after traumatic posterior hip dislocation with and without acetabular fracture[J]. Eur J Trauma Emerg Surg, 2022, 48(1): 613-619. DOI:10.1007/s00068-020-01495-x |
| [10] |
Theopold J, Armonies S, Pieroh P, et al. Nontraumatic avascular necrosis of the femoral head: arthroscopic and navigation-supported core decompression[J]. Oper Orthop Traumatol, 2020, 32(2): 107-115. DOI:10.1007/s00064-019-00643-w |
| [11] |
叶赟, 赵滨, 陈洪强, 等. 髋关节外科脱位技术在治疗早期股骨头缺血坏死中的应用观察[J]. 贵州医药, 2019, 43(1): 65-66. Ye Y, Zhao B, Chen HQ, et al. Observation on applying hip joint surgical dislocation technology for early ischemic necrosis of the femoral head[J]. Guizhou Med J, 2019, 43(1): 65-66. DOI:10.3969/j.issn.1000-744X.2019.01.021 |
| [12] |
孙海忠, 韦标方. 髋关节外科脱位打压植骨术与髓芯减压支撑植骨术治疗ARCOⅢ期股骨头缺血性坏死疗效比较[J]. 中国修复重建外科杂志, 2019, 33(5): 531-536. Sun HZ, Wei BF. Impacting bone graft via surgical hip dislocation approach versus core decompression and bone graft for avascular necrosis of femoral head at ARCO stage Ⅲ[J]. Chinese Journal of Reparative and Reconstructive Surgery, 2019, 33(5): 531-536. DOI:10.7507/1002-1892.201901047 |
| [13] |
吴剑平, 黎艺强, 李敬春, 等. 股骨头血管发育对儿童发育性髋关节脱位闭合复位术后股骨头坏死发生的影响[J]. 中华小儿外科杂志, 2021, 42(12): 1118-1123. Wu JP, Li YQ, Li JC, et al. Vascular development of femoral head and its impact on the incidence of avascular necrosis in patients with developmental dysplasia of hip after closed reduction[J]. Chin J Pediatr Surg, 2021, 42(12): 1118-1123. DOI:10.3760/cma.j.cn421158-20200608-00408 |
| [14] |
Chi ZQ, Wang S, Zhao DW, et al. Evaluating the blood supply of the femoral head during different stages of necrosis using digital subtraction angiography[J]. Orthopedics, 2019, 42(2): e210-e215. DOI:10.3928/01477447-20190118-01 |
| [15] |
Vicaș RM, Bodog FD, Fugaru FO, et al. Histopathological and immunohistochemical aspects of bone tissue in aseptic necrosis of the femoral head[J]. Rom J Morphol Embryol, 2020, 61(4): 1249-1258. DOI:10.47162/RJME.61.4.26 |
| [16] |
Zeng SL, Tu M. The lncRNA MIAT/miR-181a-5p axis regulates osteopontin (OPN)-mediated proliferation and apoptosis of human chondrocytes in osteoarthritis[J]. J Mol Histol, 2022, 53(2): 285-296. DOI:10.1007/s10735-022-10067-9 |
| [17] |
Sun PF, Kong WK, Liu L, et al. Osteopontin accelerates chondrocyte proliferation in osteoarthritis rats through the NF-κb signaling pathway[J]. Eur Rev Med Pharmacol Sci, 2020, 24(6): 2836-2842. DOI:10.26355/eurrev_202003_20647 |
| [18] |
Min SC, Shi TS, Han X, et al. Serum levels of leptin, osteopontin, and sclerostin in patients with and without knee osteoarthritis[J]. Clin Rheumatol, 2021, 40(1): 287-294. DOI:10.1007/s10067-020-05150-z |
| [19] |
Kroon FPB, Veenbrink AI, de Mutsert R, et al. The role of leptin and adiponectin as mediators in the relationship between adiposity and hand and knee osteoarthritis[J]. Osteoarthritis Cartilage, 2019, 27(12): 1761-1767. DOI:10.1016/j.joca.2019.08.003 |
| [20] |
Xiong HF, Li W, Li JJ, et al. Elevated leptin levels in temporo-mandibular joint osteoarthritis promote proinflammatory cytokine IL-6 expression in synovial fibroblasts[J]. J Oral Pathol Med, 2019, 48(3): 251-259. DOI:10.1111/jop.12819 |
 2024, Vol. 23
2024, Vol. 23


