炎性肌纤维母细胞瘤(inflammatory myofibroblastic tumor, IMT)是一种以肌成纤维细胞梭形细胞增生、浆细胞和(或)淋巴细胞明显浸润为特征的间充质肿瘤[1]。常见于肺、腹膜、肠系膜以及头颈部等,病变累及胃肠道少见[2-3]。因其发病率低,影像学及临床表现缺乏特异性,常易误诊或延误诊断,或仅于急诊手术中被发现。部分IMT具有难治性、复发性和侵袭性特点[1, 4]。本研究通过回顾性分析湖南省儿童医院普外科收治的11例胃肠道IMT患儿临床资料,结合文献总结该病的临床特征、诊断及治疗进展,以提高临床医师对该病的诊治能力。
资料与方法 一、研究对象2010年1月至2021年12月本院共收治胃肠道IMT患儿11例,其中男7例、女4例,发病年龄8个月至15岁,中位发病年龄4岁8个月。主要以腹痛、腹胀、呕吐、发热、血便及腹部肿物等症状就诊。本研究通过湖南省儿童医院医学伦理委员会审核批准(HCHLL—2023—164),患儿监护人均知情同意并签署知情同意书。
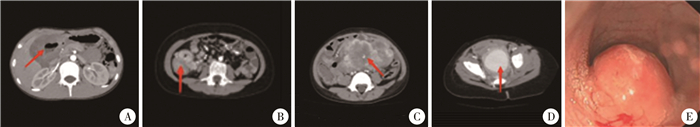
二、术前肿瘤标志物与影像学检查情况11例患儿神经元特异性希醇化酶(neuron specific enolase, NSE)和甲胎蛋白(alpha-fetoprotein, AFP)等肿瘤标志物检查均无异常。CT或MRI影像学检查显示病变涉及胃、十二指肠至直肠各段肠管,回盲部的IMT病灶弥漫性浸润明显,边界不清楚,且肠腔狭窄;合并肠套叠的IMT肠管呈层状、环状强化改变;结直肠的IMT为累及肠管内外的单发病灶,肠壁呈环形增厚,均匀强化明显。2例电子结肠镜检查见肠腔内突出肿物(肿瘤基底直径>3 cm),肠镜下难以切除。炎性肌纤维母细胞瘤患儿影像学及肠镜检查典型照片见图 1。
|
图 1 炎性肌纤维母细胞瘤患儿CT平扫+增强及肠镜检查影像 Fig.1 Imaging and colonoscopic images of IMT children 注 A:胃、十二指肠连接部IMT;B:回盲部IMT;C:空肠-回肠交界部IMT;D:直肠IMT;E:肠镜下见直肠-乙状结肠交界部肠腔内肿物;IMT:炎性肌纤维母细胞瘤 |
观察患儿治疗情况、治疗结局以及随访情况。随访时间为6~60个月,随访时间节点分别为术后1个月、3个月、6个月、1年、2年和5年,采用电话及门诊方式随访。随访时检查以腹部B超及MRI等为主,观察腹部伤口愈合情况,有无腹痛、呕吐、发热及有无肿瘤复发与转移等。免疫组化标志物检查指标包括间变性淋巴瘤激酶(anaplastic lymphoma kinase, ALK)、平滑肌肌动蛋白(smooth muscle actin, SMA)、S-100、波形蛋白(vimentin)、增殖细胞核抗原(Ki-67)、结蛋白(dsemin)、CD34以及CK蛋白。
结果11例患儿中,10例经一期手术完整切除肿瘤,其中1例术后予化疗;1例经活检确诊后未手术仅予化疗。手术时间(120±65)min,术中出血量(65±17)mL,肿瘤重量(203±45)g。11例患儿性别、年龄、临床表现、肿瘤位置、治疗方式、复发或转移、随访时间及预后情况见表 1。
| 表 1 11例胃肠道炎性肌纤维母细胞瘤患儿临床特征、治疗方式及预后 Table 1 Clinical characteristics, treatment and prognosis of 11 children with abdominal gastrointestinal IMT |
|
|
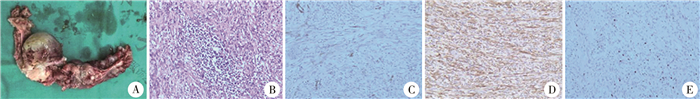
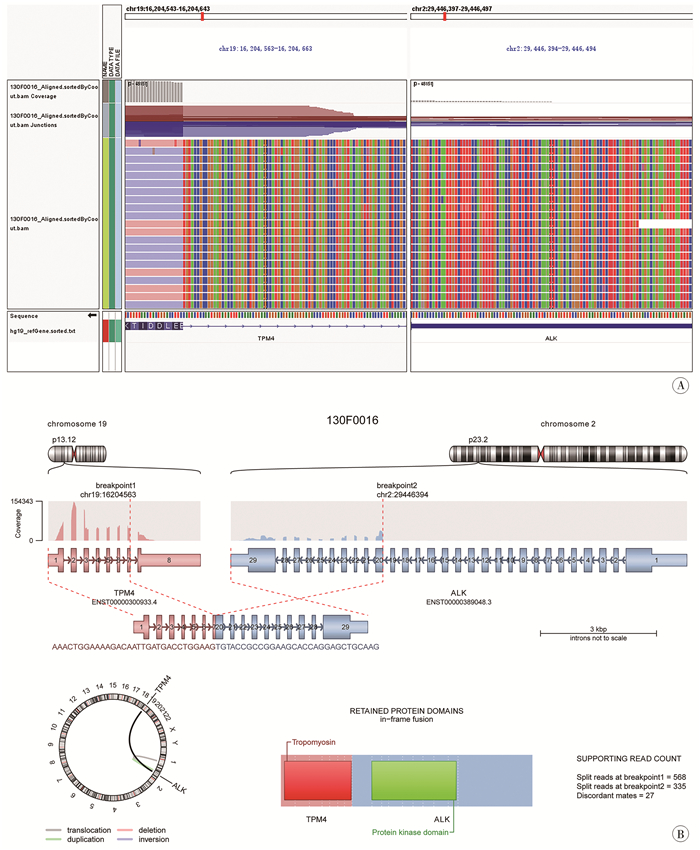
术后病理检查结果:大体标本见肿块呈椭圆或不规则分叶外观,切面呈灰白或灰黄色,外观呈囊状或束状纤维组织状;镜下见梭形细胞增生,呈编织状或席纹状,伴大量炎性细胞浸润,或玻璃样变或局灶出血。免疫组化标志物检查结果显示:ALK阳性8例,SMA阳性5例,vimentin阳性9例,desmin阳性5例。大体标本及部分病理检查结果见图 2;1例直肠IMT患儿(病例5)检测出TPM4-ALK融合,见图 3。
|
图 2 1例炎性肌纤维母细胞瘤患儿大体标本、病理及免疫组化结果 Fig.2 Gross specimen and pathological immunohistochemistry of IMT children 注 A:手术大体标本图;B:镜下见梭形细胞增生呈编织状或席纹状,伴有大量炎性细胞浸润(HE染色,×100);C:平滑肌肌动蛋白(局灶+)(HE染色,×100);D:间变性淋巴瘤激酶(+)(HE染色,×100);E:增殖细胞核抗原(5%)(HE染色,×100);IMT:炎性肌纤维母细胞瘤 |
|
图 3 1例直肠炎性肌纤维母细胞瘤TPM4-ALK融合基因IGV截图及TPM4-ALK融合基因示意图 Fig.3 TPM4-ALK Schematic diagram of fusion genes; TPM4-ALK Screenshot of IGV fusion gene 注 A:TPM4-ALK融合基因IGV截图;B:TPM4-ALK融合基因示意图;IGV:综合性基因组学可视化工具 |
IMT于1939年由Brunn等[2]首次报道,已被证实为一种真性肿瘤。2002年世界卫生组织(World Health Organization, WHO)将IMT归类为中度恶性、少数可转移的肿瘤性病变,此后IMT被视为一种独立疾病[5]。研究发现,IMT的发生与2p23染色体的ALK基因异位相关;也可由感染性疾病、异常修复、EB(Epstein-Barr, EB)病毒或特殊细菌感染、术后过度炎症反应、其他恶性肿瘤或自身免疫性疾病引起[6-7]。
IMT的人群患病率为0.04% ~0.7%,多见于儿童及青少年,无明显性别差异。本研究纳入的11例中,男7例、女4例,发病年龄8个月至15岁,中位发病年龄4岁8个月,主要以腹痛、呕吐、发热、血便及腹部肿物就诊。患儿临床症状与肿瘤占位效应及炎症浸润密切相关,并因解剖位置而异[2-3]。在胃肠道以回盲部及回肠末端多见。本组病例中,胃十二指肠IMT患儿常表现为饱腹感、呕吐;空回肠IMT患儿常以腹痛、腹胀或并发肠套叠、肠梗阻就诊;结直肠部IMT常表现为下腹疼痛、腹胀以及腹泻、大便带血等。此外,胃肠道IMT还可出现消瘦、发热、贫血等全身症状[4, 8]。
约20%的IMT患儿实验室检查发现白细胞、C反应蛋白、血沉等炎症指标升高[9-10]。虽然IMT患儿彩超及影像学检查结果无特异性,但仍然是十分必要的辅助检查[11]。本研究中,回盲部IMT患儿CT检查提示病灶弥漫性浸润明显,病灶边界不清楚,肠腔狭窄;发生肠套叠的IMT患儿CT检查见管壁呈层状、环状不均匀强化改变;结肠、直肠IMT检查提示累及肠管内外单发病灶,肠壁呈环形增厚,不均匀强化。因此在研判影像学等辅助检查结果时,如发现边界不清楚、不均匀强化及有明显侵犯邻近组织的软组织肿块,结合临床表现及实验室检查等,需考虑到IMT可能,但最终诊断仍依赖于病理检查结果[12]。
病理检查是确诊IMT的金标准,其主要特征性改变为梭形肌纤维母细胞伴炎症细胞浸润,间质水肿黏液变[4];病理组织中发现有肌样细胞间质、巨噬细胞和神经节样细胞、肿瘤脉管侵犯以及切缘浸润的IMT常提示预后不良[13-14]。免疫组化常出现ALK、Vimentin、Dsemin、SMA阳性。上皮样炎性肌纤维母细胞肉瘤(epithelioid inflammatory myofibroblastic sarcoma, eIMS)IMT的一个罕见病理亚型,临床表现为高度侵袭性生物学行为和高复发致死率。1例IMT患儿复发可能与Ki-67、ALK活性存在相关性,该患儿Ki-67增值指数为25%、ALK(3+),为本组中最高水平。但本研究样本量较小,Ki-67、ALK(3+)是否与IMT复发及预后相关,还需要多中心大样本量研究进一步验证。Chan等[15]纳入61例IMT患儿并进行长达10年的随访,发现IMT中ALK(+)常提示预后良好;但Coffin等[16]认为ALK(+)与IMT局部复发有关。也有研究发现有远处转移的患儿ALK(-),认为ALK(-)的患儿更容易发生远处转移[17]。
目前普遍认为IMT的发生与2p23染色体ALK基因易位有关,易位致使ALK与不同基因融合,从而形成致癌融合基因[6]。目前已知的RANBP2-ALK突变型eIMS肿瘤呈上皮样形态,侵袭性较强,预后差[18]。ALK常见的融合基因有NPMI、KIF5B、TPM3、TPM4、CLTC、CARS、RANBP2、ATIC等[19];ROS1、RET、NTRKI/3和IGFIR基因重排在IMT中亦有报道[19]。不同部位IMT肿瘤基因突变类型和融合基因不同:在腹部IMTs中RANBP2和CLTC基因的ALK融合发生率明显高于其他脏器;肺IMT的ROS1重排更具特征性;NTRK3和PDGFRb融合仅在胸椎IMT中检测到[20]。采用荧光原位杂交检测(fluorescence in situ hybridization, FISH)以及二代基因测序(next generation sequencing, NGS)新技术检测有无ALK重排及融合基因,对于判断IMT患儿预后、有无肿瘤复发转移等尤为重要。本组病例使用ALK分离探针,2例检出ALK基因重排,但并未接受靶向免疫治疗。因此,对于有条件的患者、诊断有争议的病例、复发性IMT以及考虑为eIMS者建议进一步行NGS检测。
儿童IMT多为良性病变,少数具有恶性潜能。手术完整切除是影响IMT预后的独立危险因素,放化疗在部分病例中初见成效[17, 21]。难以手术完整切除、多发转移的IMT及eIMS患儿可考虑新辅助化疗、术后放化疗及腹腔热灌注化疗(hyperthemic intraperitoneal chemotherapy, HIPEC)等治疗[17]。2020年欧洲儿童软组织肉瘤学会组(European pediatric Soft Tissue Sarcoma Study Group, EpSSG)指出:对于无法切除的肿瘤,化疗仍然是一种有效的选择,总体有效率可达64%[22]。目前化疗方案采取EpSSG推荐的以蒽环类/异环磷酰胺为基础,或甲氨蝶呤和长春瑞滨/长春碱为基础的化疗方案[22]。前期研究中也有抗感染或激素治疗有效的报道,但在停止用药后出现疾病进展[23]。2010年ALK抑制剂克唑替尼治疗IMT的效果得到认可,其在IMT患儿中的安全性与疗效也被证实,但有研究人员发现ALK耐药性突变、克唑替尼治疗失败的病例[24-26]。虽然目前尚无关于耐药患者二线和挽救治疗的共识,但有研究发现第二代ALK抑制剂色瑞替尼对挽救克唑替尼治疗失败或耐药的IMT患儿有效,甚至有完全缓解的个案报道[25, 27]。在2/3的ALK阴性、伴转移和(或)复发的IMT中可检测到PD-L表达,有研究认为针对PD-Lι的靶点治疗可能对上述IMT患者有效[28]。也有报道ALK抑制剂和维布妥昔单抗(一种单克隆CD30抗体)联合靶向治疗eIMS患者的生存期显著提高,但目前国际上仍未就IMT的最优治疗方案达成共识[29]。
综上所述,IMT是一类罕见间叶源性肿瘤,手术是IMT的主要治疗手段,目前临床已证实ALK抑制剂和新辅助化疗等对IMT患儿有效,但其对复发难治性IMT患儿的疗效还需通过更多前瞻性研究进行评估(尤其是耐药性IMT的挽救治疗方案);免疫靶向药物治疗手段为IMT提供了许多新思路,但仍面临诸多挑战。
利益冲突 所有作者声明不存在利益冲突
作者贡献声明 刘登辉、黄召负责文献检索;刘登辉、黎明负责论文设计;唐湘莲、向强兴负责数据收集与分析;刘登辉、周宇翔负责论文结果撰写和讨论分析;李勇负责对文章知识性内容进行审阅
| [1] |
Gros L, Dei Tos AP, Jones RL, et al. Inflammatory myofibroblastic tumour: state of the art[J]. Cancers (Basel), 2022, 14(15): 3662. DOI:10.3390/cancers14153662 |
| [2] |
Brunn H. Two interesting benign lung tumors of contradictory histopathology: remarks on the necessity for maintaining the chest tumor registry[J]. J Thorac Surg, 1939, 9(2): 119-131. DOI:10.1016/S0096-5588(20)32030-4 |
| [3] |
Surabhi VR, Chua S, Patel RP, et al. Inflammatory myofibroblastic tumors: current update[J]. Radiol Clin North Am, 2016, 54(3): 553-563. DOI:10.1016/j.rcl.2015.12.005 |
| [4] |
陈欣媛, 李智. 儿童炎性肌纤维母细胞瘤诊治进展[J]. 临床小儿外科杂志, 2023, 22(3): 296-300. Chen XY, Li Z. Updates in diagnosis and treatment of inflammatory myofibroblastic tumor in children[J]. J Clin Ped Sur, 2023, 22(3): 296-300. DOI:10.3760/cma.j.cn101785-202204073-018 |
| [5] |
Jo VY, Fletcher CDM. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition[J]. Pathology, 2014, 46(2): 95-104. DOI:10.1097/PAT.0000000000000050 |
| [6] |
Li YP, Han WW, He LJ, et al. Inflammatory myofibroblastic tumor after treatment of Wilms tumor in a 6-year-old boy: a case report and literature review[J]. Urology, 2021, 149: e25-e28. DOI:10.1016/j.urology.2020.11.012 |
| [7] |
DongYH, Zahid KR, Han YD, et al. Treatment of pediatric inflammatory myofibroblastic tumor: the experience from China Children's Medical Center[J]. Children (Basel), 2022, 9(3): 307. DOI:10.3390/children9030307 |
| [8] |
Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO classification of soft tissue tumours: news and perspectives[J]. Pathologica, 2021, 113(2): 70-84. DOI:10.32074/1591-951X-213 |
| [9] |
Anderson WJ, Doyle LA. Updates from the 2020 World Health Organization classification of soft tissue and bone tumours[J]. Histopathology, 2021, 78(5): 644-657. DOI:10.1111/his.14265 |
| [10] |
胡嘉健, 黄一晋, 韩建宇, 等. 小儿腹部炎性肌纤维母细胞瘤诊治探讨[J]. 临床小儿外科杂志, 2020, 19(4): 336-341. Hu JJ, Huang YJ, Han JY, et al. Abdominal inflammatory myofibroblastic tumors in children: a clinical diagnosis and treatment review[J]. J Clin Ped Sur, 2020, 19(4): 336-341. DOI:10.3969/j.issn.1671-6353.2020.04.011 |
| [11] |
Torres US, Matsumoto C, Maia DR, et al. Computed tomography and magnetic resonance imaging findings of inflammatory pseudotumors in the abdomen and pelvis: current concepts and pictorial review[J]. Semin Ultrasound CT MR, 2018, 39(2): 220-229. DOI:10.1053/j.sult.2017.09.005 |
| [12] |
Liu B, Xu JL, Wang JX, et al. Computed tomography appearance of inflammatory myofibroblastic tumor in the abdomen: CT features and pathologic correlation[J]. Int J Clin Exp Med, 2015, 8(9): 16745-16755. |
| [13] |
Bennett JA, Nardi V, Rouzbahman M, et al. Inflammatory myofibroblastic tumor of the uterus: a clinicopathological, immunohistochemical, and molecular analysis of 13 cases highlighting their broad morphologic spectrum[J]. Mod Pathol, 2017, 30(10): 1489-1503. DOI:10.1038/modpathol.2017.69 |
| [14] |
Devereaux KA, Fitzpatrick MB, Hartinger S, et al. Pregnancy-associated inflammatory myofibroblastic tumors of the uterus are clinically distinct and highly enriched for TIMP3-ALK and THBS1-ALK fusions[J]. Am J Surg Pathol, 2020, 44(7): 970-981. DOI:10.1097/PAS.0000000000001481 |
| [15] |
Chan JK, Cheuk W, Shimizu M. Anaplastic lymphoma kinase expression in inflammatory pseudotumors[J]. Am J Surg Pathol, 2001, 25(6): 761-768. DOI:10.1097/00000478-200106000-00007 |
| [16] |
Coffin CM, Hornick JL, Fletcher CDM. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases[J]. Am J Surg Pathol, 2007, 31(4): 509-520. DOI:10.1097/01.pas.0000213393.57322.c7 |
| [17] |
Nakano K. Inflammatory myofibroblastic tumors: recent progress and future of targeted therapy[J]. Jpn J Clin Oncol, 2023, 53(10): 885-892. DOI:10.1093/jjco/hyad074 |
| [18] |
Zhu Y, Ding Y, Song GX, et al. Clinicopathological features of inflammatory myofibroblastic tumor[J]. Chin J Pathol, 2021, 50(3): 194-200. DOI:10.3760/cma.j.cn112151-20200806-00627 |
| [19] |
Siemion K, Reszec-Gielazyn J, Kisluk J, et al. What do we know about inflammatory myofibroblastic tumors?-A systematic review[J]. Adv Med Sci, 2022, 67(1): 129-138. DOI:10.1016/j.advms.2022.02.002 |
| [20] |
Preobrazhenskaya EV, Suleymanova AM, Bizin IV, et al. Spectrum of kinase gene rearrangements in a large series of paediatric inflammatory myofibroblastic tumours[J]. Histopathology, 2023, 83(1): 109-115. DOI:10.1111/his.14912 |
| [21] |
Biswas R, Halder A, Gangopadhyay M, et al. Inflammatory myofibroblastic tumor of maxillary sinus successfully treated with radiotherapy and corticosteroid: report of a rare case[J]. J Egypt Natl Canc Inst, 2020, 32(1): 26. DOI:10.1186/s43046-020-00038-0 |
| [22] |
Casanova M, Brennan B, Alaggio R, et al. Inflammatory myofibroblastic tumor: The experience of the European pediatric Soft Tissue Sarcoma Study Group (EpSSG)[J]. Eur J Cancer, 2020, 127: 123-129. DOI:10.1016/j.ejca.2019.12.021 |
| [23] |
Panigada S, Sacco O, Girosi D, et al. Corticosteroids may favor proliferation of thoracic inflammatory myofibroblastic tumors[J]. Pediatr Pulmonol, 2014, 49(3): E109-E111. DOI:10.1002/ppul.22977 |
| [24] |
Butrynski JE, D'Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor[J]. N Engl J Med, 2010, 363(18): 1727-1733. DOI:10.1056/NEJMoa1007056 |
| [25] |
Trahair T, Gifford AJ, Fordham A, et al. Crizotinib and surgery for long-term disease control in children and adolescents with ALK-positive inflammatory myofibroblastic tumors[J]. JCO Precis Oncol, 2019, 3: 1-11. DOI:10.1200/PO.18.00297 |
| [26] |
Mansfield AS, Murphy SJ, Harris FR, et al. Chromoplectic TPM3-ALK rearrangement in a patient with inflammatory myofibroblastic tumor who responded to ceritinib after progression on crizotinib[J]. Ann Oncol, 2016, 27(11): 2111-2117. DOI:10.1093/annonc/mdw405 |
| [27] |
Mittal A, Gupta A, Rastogi S, et al. Near-complete response to low-dose ceritinib in recurrent infantile inflammatory myofibroblastic tumour[J]. Ecancermedicalscience, 2021, 15: 1215. DOI:10.3332/ecancer.2021.1215 |
| [28] |
Cottrell TR, Duong AT, Gocke CD, et al. PD-L1 expression in inflammatory myofibroblastic tumors[J]. Mod Pathol, 2018, 31(7): 1155-1163. DOI:10.1038/s41379-018-0034-6 |
| [29] |
Fordham AM, Xie JH, Gifford AJ, et al. CD30 and ALK combination therapy has high therapeutic potency in RANBP2-ALK-rearranged epithelioid inflammatory myofibroblastic sarcoma[J]. Br J Cancer, 2020, 123(7): 1101-1113. DOI:10.1038/s41416-020-0996-2 |
 2024, Vol. 23
2024, Vol. 23


