2. 湖南省儿童医院药学部, 长沙 410007
2. Department of Pharmacy, Hunan Children's Hospital, Changsha 410007, China
具核梭杆菌属于梭杆菌属,是一种革兰氏染色阴性专性厌氧菌,是人类口腔及胃肠道重要微生物,它既可以表现为共生菌,也可以表现为机会致病菌和致癌菌,参与多种口腔疾病(如牙周病、口腔癌)的发生发展,与直肠癌关系密切,还可以引起多种感染性与非感染性疾病,如心包炎、脑脓肿、肝脓肿、阑尾炎等[1-6]。具核梭杆菌所致骨髓炎临床上罕见,目前国内尚无相关文献报道。检索国外文献共报道具核梭杆菌所致儿童骨髓炎10例,主要为4岁以上男童,病程呈亚急性,疼痛多集中在病变局部,累及邻近关节者有关节活动受限[7]。本文回顾性分析湖南省儿童医院骨科近期收治的1例具核梭杆菌致股骨远端骨骺骨髓炎患儿诊疗经过,并进行相关文献综述,以提高临床医师对于该病的认识。
资料与方法 一、本院收治患儿诊疗情况患儿,男性,就诊时年龄4岁2个月,因“右膝部疼痛、肿胀、跛行1月余”入院。查体:轻度跛行步态,右膝关节皮肤颜色正常、轻度肿胀。右侧股骨远端外侧及后侧压痛。右膝关节轻度痛性屈伸活动受限,浮髌试验阴性。髋关节和踝关节感觉、运动及血运无明显异常。血液白细胞计数、血沉和C反应蛋白均正常。术前膝关节X线检查提示右侧股骨远端骨骺外侧密度稍减低、欠均匀,未见骨膜反应和死骨征(图 1A)。膝关节CT提示右侧股骨远端骨骺外侧部分骨质破坏,累及骺板,大小约2.1 cm×1.1 cm×1.0 cm,边缘稍硬化,未见明显骨膜反应。正位骨骺破坏缺损区上下径及横径、股骨骨骺上下径及横径之比分别为72%和48%;侧位为70%和42%。周围软组织肿胀(图 1B)。膝关节MRI:右侧股骨远端及骨骺大片状异常信号影及周围软组织改变(图 1C)。
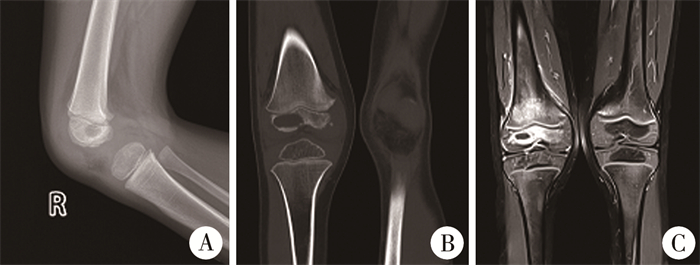
|
图 1 1例4岁2个月具核梭杆菌致股骨远端骨骺骨髓炎患儿影像学改变 Fig.1 Radiographic changes in a 50-month-old child with Fusobacterium nucleatum osteomyelitis of distal femur 注 A:X线检查提示右侧股骨远端骨骺外侧部密度稍减低;B:CT提示右侧股骨远端骨骺外侧见骨质破坏区;C:MRI提示右侧股骨远端及骨骺大片状异常信号影及周围软组织改变 |
入院诊断:①右侧膝关节内股骨远侧骨骺病变,考虑为软骨母细胞瘤?骨骺骨髓炎?恶性肿瘤?②右侧膝关节积液,考虑为化脓性关节炎?反应性关节炎?需鉴别和排除骨样骨瘤、尤文肉瘤、骨肉瘤、骨巨细胞瘤、白血病、朗格汉斯细胞组织增生症等[7-9]。
治疗方法:静脉全身麻醉下行右侧股骨远端外侧骨骺内病灶切开活检术。术中自右膝关节穿刺抽出清亮、透明、黏稠关节液1 mL,排除急性化脓性关节炎。于股骨远端骨骺外侧关节软骨非负重区行横“U”形开窗,大小约6 mm×3 mm,缝合固定牵拉外翻软骨窗,见白色米汤样浑浊液体流出,将注射器插入病灶并收集2 mL液体标本,见大量白色絮状物;探查见股骨远端骨骺内病灶为空腔,大小约2 cm×2 cm×1.5 cm,刮取少量淡黄色液化黏稠软组织、灰白色膜样组织及少量血凝块。将术中留取的液体标本及病理组织送病理检查、细菌培养及病原微生物高通量宏基因检测二代测序技术(next generation sequencing, NGS)。
患儿病理检查结果符合慢性化脓性炎症改变,NGS提示为G-梭杆菌属具核梭杆菌,相对丰度95.24%。予克林霉素40 mg·kg-1·d-1静脉滴注,每8 h给药一次。术后第11天行右侧膝关节内股骨远端骨骺内感染病灶清理冲洗,将克林霉素0.6 g与10 mL硫酸钙-磷酸钙人工骨粉混合成膏状缓释载体,取5 mL植入病灶内,缝合修复软骨窗。
二、文献检索方法检索Pubmed、万方医学网和中国知网自1970年1月至2023年8月公开发表的文献,中文检索词为“具核梭杆菌”、“骨髓炎”,英文检索词为“Fusobacterium nucleatum”、“osteomyelitis”。文献纳入标准:具核梭杆菌所致骨髓炎的病例报告或临床研究型文献。文献排除标准:①文献报告数据不全,资料不齐;②综述、信件、会议摘要或同一数据来源的文献。
结果患儿出院时右股骨远端无明显疼痛,膝关节屈伸活动基本正常,各项炎症指标阴性。出院后继续口服克林霉素棕榈酸酯颗粒7周,剂量为20 mg·kg-1·d-1,每8 h给药一次。每2周随诊1次,至术后10周。术后10周随访,患儿无发热,右侧股骨远端及膝关节无疼痛,右侧股骨远端切口愈合良好,股骨远端无明显压痛,右膝肿胀消退,右膝关节主动屈伸130°~0°。X线检查提示右侧股骨远端骨骺外侧部分可见高密度人工骨,较前稍吸收,密度较前稍减低,右侧股骨远端骨骺及干骺端密度欠均匀,暂未见股骨远端骺板骨桥(图 2)。根据患儿局部以及全身症状、影像学表现,确定骨髓炎已治愈。

|
图 2 具核梭杆菌感染致股骨远端骨骺骨髓炎患儿术后10周X线检查见局部高密度人工骨影,未见股骨远端骺板骨桥 Fig.2 Follow-up radiographic examination at Week 10 post-operation in a child with osteomyelitis caused by Fusobacterium nucleatum.Localized high-density artificial bone shadow had no evidence of femoral distal metaphysis bone bridge 注 A:右膝关节正位X线片;B:右膝关节侧位X线片 |
本研究共纳入符合纳入与排除标准文献8篇(表 1),均为外文文献,共报道具核梭杆菌感染致儿童骨髓炎10例,患儿年龄4~12岁,其中骨骺骨髓炎3例,亚急性骨髓炎7例。10例中合并化脓性关节炎7例;经病灶组织和关节液厌氧菌培养确诊9例,经PCR确诊1例。10例均予对厌氧菌敏感的抗生素(β-内酰胺类药物、甲硝唑、克林霉素)治疗,其中7例行病灶穿刺。平均药物治疗时间8.6周,至末次随访时,患儿均症状消失,运动功能接近正常。
| 表 1 文献报道中具核梭杆菌致儿童骨髓炎病例资料 Table 1 Clinical profiles of children with osteomyelitis caused by Fusobacterium nucleatum |
|
|
梭杆菌属是革兰氏阴性厌氧杆菌,可在健康人的口咽和胃肠道分离出来[1]。在不同梭杆菌种属中,最常从临床标本中分离出来的是具核梭杆菌。儿童梭杆菌属骨髓炎最常见于颅骨和面部骨骼,与中耳炎、乳突炎、鼻窦炎、创伤和口腔感染有关[8-9]。
梭杆菌属骨髓炎的治疗包括抗生素治疗和适时手术干预。最常用的抗生素包括β-内酰胺类药物、克林霉素和甲硝唑。青霉素仍然是梭杆菌属感染的有效治疗药物,但有研究表明约9%的梭杆菌株对青霉素产生了耐药性[18]。对于β-内酰胺类药物过敏的患儿,可以考虑克林霉素或甲硝唑。甲硝唑耐药临床罕见,但其容易引起恶心,因而限制了在儿童中的应用[18]。克林霉素适用于敏感厌氧菌及需氧菌(肺炎链球菌、A组溶血性链球菌及金黄色葡萄球菌等)感染,且其在骨组织中浓度高[19]。但也有学者报道梭杆菌属对克林霉素有一定的耐药性[20]。
关于抗生素治疗的确切持续时间取决于疾病严重程度、临床反应和炎症标志物情况,同时应根据病灶侵犯范围及程度选择合适的手术干预方式。本研究中,文献报告的7例骨髓炎和3例骨骺骨髓炎患儿病灶骨质缺损范围小或无骨质缺损。本院收治的患儿股骨远端骨骺破坏范围广泛,且累及骺板,骨骺骨髓炎可出现病情迁延,进而导致慢性骨髓炎,骺板和骨骺进一步破坏,引发病理性骨折,甚至出现继发性骨关节生长发育障碍和畸形等。
通常急性血源性骨髓炎在脓肿形成或抗生素治疗48~72 h无效的情况下才考虑手木治疗[21]。主要手术方式为骨髓炎病灶开窗减压加病灶清理和灌洗引流。对于慢性骨髓炎,较多采用抗生素和缓释载体人工骨植入髓腔的方法[22]。缓释载体中的抗生素可以维持药效4周左右[23-24]。本院收治的患儿因骨骺感染导致脓肿和囊腔破坏,病程超过1个月,系亚急性骨髓炎,因此,术中清除病灶坏死液化组织后,在空腔内植入克林霉素+硫酸钙-磷酸钙人工骨粉制成的抗生素人工骨缓释体,不但有利于骨质缺损的修复,人工骨中抗生素的缓释还可以持续杀灭局部病灶内细菌,防止复发。
因厌氧菌生长缓慢,分离和鉴定困难,以及耐药性和多样化的微生物性质,导致厌氧菌感染的诊断和治疗变得复杂[25]。梭杆菌属为专性厌氧菌,在手术取样并将其接种到维持厌氧条件的培养基时,保持生物体活力变得困难。同时,由于厌氧培养的敏感性低,建议使用NGS检测细菌性骨髓炎中的微生物。NGS超越了传统微生物学检查的局限性,为分析样品中全谱微生物,甚至宿主的基因组或转录组提供了机会[26]。NGS具有多项优势,包括不依赖细菌培养,能够检测所有微生物,以及可以直接从临床样本中鉴定罕见、新型、难以检测以及合并感染的病原体等。当然,NGS也有一定的局限,每个样本至少需要读取20万次,且价格昂贵。但为了尽早确定病原体,我们认为应该更加重视儿童骨髓炎中厌氧菌的检测。
具核梭杆菌感染引起的儿童骨髓炎诊断困难的原因可能与未获取有效病变组织和未进行厌氧菌培养有关。针对病灶较小、病理组织稀少、难以预测和诊断的儿童骨髓炎,可以行NGS检测、病理检查以及需氧、厌氧菌培养,以帮助定性诊断,并根据病原学证据制订有效的抗生素治疗方案。对于骨髓炎导致的髓腔内外脓肿和骨缺损,可以根据感染病灶位置、范围、脓液多少、破坏程度,采用穿刺引流或切开病灶清理引流、局部抗生素和人工骨缓释载体植入的方案。骨髓炎的治疗应力争快速控制感染并修复骨缺损,避免感染扩散或反复发作、病理性骨折及病理性骺板破坏等导致严重后果。
总之,具核梭杆菌所致儿童骨髓炎临床罕见,厌氧培养及NGS是确诊具核梭杆菌感染所致骨髓炎的重要手段。病灶切开引流+敏感抗生素治疗是该病的有效治疗手段。对骨质缺损较多病例,病灶清理、灌洗引流后采取敏感抗生素、人工骨缓释载体抗生素植入的方案可以获得满意疗效。
利益冲突 所有作者声明不存在利益冲突
作者贡献声明 伍江雁、胡雄科负责课题设计、数据收集、文章撰写与修改;曾凌嵘、向晓琴、吴丽霞负责标本收集和文献检索;梅海波负责对全文知识性内容进行审阅
| [1] |
Brennan CA, Garrett WS. Fusobacterium nucleatum-symbiont, opportunist and oncobacterium[J]. Nat Rev Microbiol, 2019, 17(3): 156-166. DOI:10.1038/s41579-018-0129-6 |
| [2] |
毛旭虎, 童亚楠. 具核梭杆菌研究进展[J]. 第三军医大学学报, 2019, 41(19): 1867-1872. Mao XH, Tong YN. Research advances of Fusobacterium nucleatum[J]. J Third Mil Med Univ, 2019, 41(19): 1867-1872. DOI:10.16016/j.1000-5404.201908133 |
| [3] |
Truant AL, Menge S, Milliorn K, et al. Fusobacterium nucleatum pericarditis[J]. J Clin Microbiol, 1983, 17(2): 349-351. DOI:10.1128/jcm.17.2.349-351.1983 |
| [4] |
Han XY, Weinberg JS, Prabhu SS, et al. Fusobacterial brain abscess: a review of five cases and an analysis of possible pathogenesis[J]. J Neurosurg, 2003, 99(4): 693-700. DOI:10.3171/jns.2003.99.4.0693 |
| [5] |
Toyoshima H, Tanigawa M, Ishiguro C, et al. Vertebral osteomyelitis caused by Fusobacterium nucleatum with an associated asymptomatic liver abscess in an immunocompetent adult: a case report and literature review[J]. ID Cases, 2023, 32: e01754. DOI:10.1016/j.idcr.2023.e01754 |
| [6] |
Swidsinski A, Dörffel Y, Loening-Baucke V, et al. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum[J]. Gut, 2011, 60(1): 34-40. DOI:10.1136/gut.2009.191320 |
| [7] |
Fan ZX, Tang PZ, Li C, et al. Fusobacterium nucleatum and its associated systemic diseases: epidemiologic studies and possible mechanisms[J]. J Oral Microbiol, 2022, 15(1): 2145729. DOI:10.1080/20002297.2022.2145729 |
| [8] |
李雅琴, 李论, 徐涛涛, 等. 儿童亚急性骨髓炎的研究进展[J]. 临床小儿外科杂志, 2023, 22(5): 485-489. Li YQ, Li L, Xu TT, et al. Research advances of subacute osteomyelitis in children[J]. J Clin Ped Sur, 2023, 22(5): 485-489. DOI:10.3760/cma.j.cn101785-202211063-016 |
| [9] |
Almuzam S, Howard‐Jones AR, Birke O, et al. Subacute osteomyelitis caused by Fusobacterium nucleatum in a healthy child[J]. J Paediatr Child Health, 2021, 57(12): 2010-2011. DOI:10.1111/jpc.15408 |
| [10] |
Gregory SW, Boyce TG, Larson AN, et al. Fusobacterium nucleatum osteomyelitis in 3 previously healthy children: a case series and review of the literature[J]. J Pediatric Infect Dis Soc, 2015, 4(4): e155-e159. DOI:10.1093/jpids/piv052 |
| [11] |
Kroon E, Arents NA, Halbertsma FJ. Septic arthritis and osteomyelitis in a 10-year-old boy, caused by Fusobacterium nucleatum, diagnosed with PCR/16S ribosomal bacterial DNA amplification[J]. BMJ Case Rep, 2012, 2012: bcr1220115335. DOI:10.1136/bcr.12.2011.5335 |
| [12] |
Murray SJ, Lieberman JM. Fusobacterium osteomyelitis in a child with sickle cell disease[J]. Pediatr Infect Dis J, 2002, 21(10): 979-981. DOI:10.1097/00006454-200210000-00020 |
| [13] |
Brook I. Fusobacterial infections in children[J]. J Infect, 1994, 28(2): 155-165. DOI:10.1016/s0163-4453(94)95600-6 |
| [14] |
Beauchamp RD, Cimolai N. Osteomyelitis of the pelvis due to Fusobacterium nucleatum[J]. Can J Surg, 1991, 34(6): 618-620. |
| [15] |
Brook I. Two cases of diskitis attributable to anaerobic bacteria in children[J]. Pediatrics, 2001, 107(2): E26. DOI:10.1542/peds.107.2.e26 |
| [16] |
Budd E, Johnson DS, Thomas E, et al. Subacute osteomyelitis of the femur due to Fusobacterium nucleatum in a 7-year-old boy[J]. Pediatr Infect Dis J, 2015, 34(3): 324-326. DOI:10.1097/INF.0000000000000558 |
| [17] |
Lewis RP, Sutter VL, Finegold SM. Bone infections involving anaerobic bacteria[J]. Medicine (Baltimore), 1978, 57(4): 279-305. DOI:10.1097/00005792-197807000-00001 |
| [18] |
Aldridge KE, Ashcraft D, Cambre K, et al. Multicenter survey of the changing in vitro antimicrobial susceptibilities of clinical isolates of Bacteroides fragilis group, Prevotella, Fusobacterium, Porphyromonas, and Peptostreptococcus species[J]. Antimicrob Agents Chemother, 2001, 45(4): 1238-1243. DOI:10.1128/AAC.45.4.1238-1243.2001 |
| [19] |
中国医药教育协会感染疾病专业委员会. 抗菌药物药代动力学/药效学理论临床应用专家共识[J]. 中华结核和呼吸杂志, 2018, 41(6): 409-446. Specialty Committee of Infection Diseases, Chinese Medical Education Association. Consensus of Pharmacokinetics/Pharmacodynamics and Clinical Application of Antimicrobial Agents[J]. Chin J Tuberc Respir Dis, 2018, 41(6): 409-446. DOI:10.3760/cma.j.issn.1001-0939.2018.06.004 |
| [20] |
Ackermann G, Schaumann R, Pless B, et al. Comparative activity of moxifloxacin in vitro against obligately anaerobic bacteria[J]. Eur J Clin Microbiol Infect Dis, 2000, 19(3): 228-232. DOI:10.1007/s100960050465 |
| [21] |
陶锐, 覃承诃, 方佳, 等. 儿童急性血源性骨髓炎的诊治进展[J]. 中华创伤骨科杂志, 2020, 22(9): 818-823. Tao R, Qin CH, Fang J, et al. Diagnostic and therapeutic advances of acute hematogenous osteomyelitis in children[J]. Chin J Orthop Trauma, 2020, 22(9): 818-823. DOI:10.3760/cma.j.cn115530-20200329-00214 |
| [22] |
AndreacchioA, Alberghina F, Paonessa M, et al. Tobramycin-impregnated calcium sulfate pellets for the treatment of chronic osteomyelitis in children and adolescents[J]. J Pediatr Orthop B, 2019, 28(3): 189-195. DOI:10.1097/BPB.0000000000000517 |
| [23] |
McKee MD, Li-Bland EA, Wild LM, et al. A prospective, randomized clinical trial comparing an antibiotic-impregnated bioabsorbable bone substitute with standard antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis and infected nonunion[J]. J Orthop Trauma, 2010, 24(8): 483-490. DOI:10.1097/BOT.0b013e3181df91d9 |
| [24] |
Luo SC, Jiang TM, Yang YN, et al. Combination therapy with vancomycin-loaded calcium sulfate and vancomycin-loaded PMMA in the treatment of chronic osteomyelitis[J]. BMC Musculoskelet Disord, 2016, 17(1): 502. DOI:10.1186/s12891-016-1352-9 |
| [25] |
Boyanova L, Markovska R, Yordanov D, et al. Anaerobes in specific infectious and noninfectious diseases: new developments[J]. Anaerobe, 2023, 81: 102714. DOI:10.1016/j.anaerobe.2023.102714 |
| [26] |
Chiu CY, Miller SA. Clinical metagenomics[J]. Nat Rev Genet, 2019, 20(6): 341-355. DOI:10.1038/s41576-019-0113-7 |
 2023, Vol. 22
2023, Vol. 22


