大鼠去分化脂肪(dedifferentiated fat, DFAT)细胞是由成熟白色脂肪细胞去分化得到不含脂滴的多能干细胞,具有多种分化潜能。因其同质性好,从少量脂肪组织即可获得,且获取细胞时不需考虑患者年龄等特点,DFAT细胞已成为生物工程和再生医学领域重要的多能干细胞来源[1-2]。DFAT细胞的原代培养方法有天花板培养法和滤网培养法,前者为国内外大多数学者应用的方法,后者是Jumabay等[3]报道的一种新方法[4]。基于当前多能干细胞的临床需求,我们比较了两种培养方法,以获得更优的选择。
资料与方法 一、实验动物及主要试剂① 实验动物:SD(Sprague Dawley)大鼠(4~6周、130~170 g)8只,雌雄不限,由中国医科大学附属盛京医院动物实验中心提供,许可证号:SYXK(辽)2017-0004。研究符合所在单位实验动物伦理委员会制定的伦理标准。②主要试剂:高糖达尔伯克改良伊格尔(Dulbecco's modified eagle medium, DMEM)培养基、青霉素及链霉素溶液/双抗、0.25%胰酶、混合营养物F-12 DMEM (nutrient mixture F-12, F12-DMEM)培养基、胎牛血清(fetal bovine serum, FBS)、Ⅰ型胶原酶、不含乙二胺四乙酸(ehylenediaminetetraacetic acid, EDTA)的胰酶、小鼠FcγRⅡ(CD32)抗体、仓鼠藻红蛋白标记整合素β1(phycoerythrin conjugated integrin beta 1, CD29-PE)抗体、小鼠异硫氰酸荧光素标记白细胞共同抗原(fluorescein isothiocyanate conjugated leukocyte common gntigen, CD45-FITC)抗体、小鼠藻红蛋白标记血小板-内皮细胞粘附分子(phycoerythrin conjugated platelet endothelial cell adhesion molecule-1, CD31-PE)抗体、小鼠别藻蓝蛋白标记thy-1(allophycocyanin conjugated thy-1, CD90-APC)抗体、兔抗大鼠SMMHC多克隆抗体、小鼠抗大鼠αSMA单克隆抗体、Alexa Fluor 488缀合驴抗小鼠二抗、Alexa Fluor 594缀合驴抗兔二抗、转化生长因子β1(transforming growth factor beta1, TGF-β1)、4%多聚甲醛、聚乙二醇辛基苯基醚(2-(2-[4-(1, 1, 3, 3-Tetramethylbutyl)phenoxy]ethoxy)ethanol, Triton X-100)、磷酸缓冲盐溶液(phosphate buffer saline, PBS)。
二、研究方法 (一) 成熟脂肪细胞的分离SD大鼠处死后,消毒皮肤, 取下腹部正中切口,切开皮肤,获取腹股沟皮下脂肪垫,置于预冷的含1%双抗的PBS中,解剖显微镜下去除脂肪组织中血管、淋巴结等组织,转至超净台。PBS洗涤3次,用眼科剪剪成0.5~1 mm3组织块,加入等体积0.1%Ⅰ型胶原酶,37℃水浴振荡消化60~90 min,至形成乳糜样浓稠液体。加入等体积完全培养基(高糖DMEM培养基+20%FBS+1%双抗)终止消化,100目滤网过滤,135 g离心5 min,取上层乳脂层。完全培养基洗涤3次后重悬细胞。
(二) DFAT细胞的原代培养1. 天花板培养法:按照Sugihara等[4]的方法,取0.5×106~1×106个成熟脂肪细胞,接种于装满完全培养基的培养面积为25 cm2密封培养瓶中,翻转培养瓶倒置于37℃、5%CO2孵箱中。7 d后翻转培养瓶并换液,至细胞呈梭形,生长融合率达约90%时,传代,每2~3天换液一次。
2. 滤网培养法:按Jumabay等[3]的方法,将消化洗涤后的上层乳脂层在放有完全培养基的30 mm培养皿中预培养24 h。取30~50 μL上层乳脂层加入放有完全培养基和70 μm滤网的六孔板单孔,置于37℃、5%CO2孵箱中,孵育至六孔板底部约有30%成纤维样细胞时取出滤网,至细胞呈梭形,生长融合率达约90%时传代,每2~3天换液一次。
3. 细胞形态学观察:倒置相差显微镜(Eclipse Ti, Nikon公司)下观察细胞生长情况,采集图像。
4. 流式细胞术检测DFAT细胞的表面抗原表型:取生长良好、融合率达90%的第3代DFAT细胞,在不含EDTA的胰酶消化后收集细胞,每管1×106个细胞,加入FcR阻断剂4℃孵育10 min。使用的流式抗体有:Hamster CD29-PE 5 μL、Mouse CD45-FITC 2 μL、Mouse CD31-PE 5 μL和Mouse CD90-APC 5 μL,4℃避光孵育20 min。用流式细胞检测分析仪(FACScalibur, BD公司)检测细胞,并用FlowJo软件分析细胞表面标志物表达情况。
5. 诱导DFAT细胞向平滑肌细胞分化:将5×104个第三代DFAT细胞接种在六孔板中,加入完全培养基,2 d后更换为含有5%FBS和5 ng/mL TGF-β1的培养基,诱导3周后,更换为诱导完全培养基(F12-DMEM培养基+10%FBS+1%双抗)[5]。
6. 细胞免疫荧光:将待测细胞接种于细胞爬片,用4%多聚甲醛固定,0.1%tritonX-100通透,5%山羊血清封闭,加一抗(αSMA 1 ∶ 200,SMMHC 1 ∶ 25)4℃过夜,加荧光二抗(1 ∶ 200),37℃孵育1 h,DAPI避光孵育3 min,抗荧光淬灭封片剂封片,荧光显微镜(Eclipse NI, Nikon公司)下观察、采集图像。
三、统计学处理采用SPSS 25.0进行统计分析。天花板培养法和滤网培养法培养的DFAT细胞生长至首次传代时间、流式细胞分析表面抗原标志物阳性细胞率和体外诱导后αSMA阳性细胞率以x±s表示,组间比较采用独立样本t检验;天花板培养法和滤网培养法培养的DFAT细胞生长至传代所需培养基量以M(Q1,Q3)表示,组间比较采用Mann-Whitney U检验。P < 0.05为差异有统计学意义。
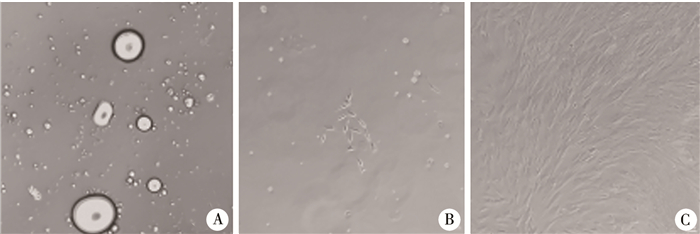
结果 一、DFAT细胞形态学观察天花板培养法培养中,将成熟脂肪细胞接种在装满完全培养基的密封培养瓶,于倒置相差显微镜下见培养瓶底部分布有大小不等的内含脂滴的单眼脂肪细胞,后脂滴消失,细胞转变为成纤维样细胞,DFAT细胞呈梭形、峰谷样排列,见图 1。
|
图 1 天花板培养法培养的DFAT细胞的形态变化(Bar=100 μm) Fig.1 Morphological changes in DFAT cells cultured by ceiling culture (Bar=100 μm) 注 A:培养第2天,见大小不等的单房脂肪细胞,内含一个大脂滴;B:培养第5天,见少量成纤维样细胞;C:培养第12天,见成纤维样细胞呈梭形、峰谷样排列 |
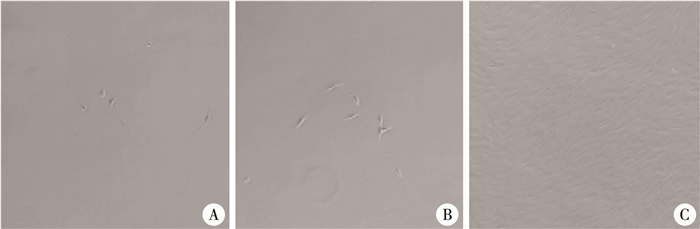
滤网培养法培养中,于倒置相差显微镜下接种2 d后可见六孔板底部开始有成纤维样细胞散在分布,后成纤维样细胞数量增加,DFAT细胞呈梭形、峰谷样排列,见图 2。
|
图 2 滤网培养法培养的DFAT细胞的形态变化(Bar=100 μm) Fig.2 Morphological changes in DFAT cells cultured by insert culture(Bar=100 μm) 注 A:培养第2天,见少量成纤维样细胞;B:培养第5天,见成纤维样细胞数较前增多;C:培养第12天,见成纤维样细胞呈梭形、峰谷样排列 |
天花板培养法和滤网培养法培养DFAT细胞生长至首次传代的时间分别为(16.00±1.41)d和(16.25±0.96)d,差异无统计学意义(t=-0.293,P=0.780)。天花板培养法和滤网培养法培养DFAT细胞生长至首次传代所需完全培养基分别为93.75(90.00,93.75)mL和48.50(48.50,48.50)mL,差异有统计学意义(U=0.000,P=0.011)。
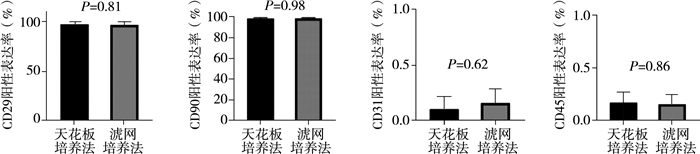
三、流式细胞分析结果天花板培养法和滤网培养法培养的DFAT细胞表面抗原标志物均表达为CD29+、CD90+、CD31-、CD45-。天花板培养法和滤网培养法培养的DFAT细胞流式细胞分析结果显示:阳性细胞百分数分别为CD29+(97.63±3.15)%,(96.93±3.61)%;CD90+(98.63±0.83)%,(98.65±0.84)%;CD31+(0.11±0.12)%,(0.16±0.13)%;CD45+(0.18±0.11)%,(0.16±0.10)%;差异均无统计学意义(P>0.05),见图 3。
|
图 3 天花板培养法和滤网培养法培养的DFAT细胞流式细胞分析结果 Fig.3 Comparison of the results of flow cytometry of DFAT cells cultured by ceiling culture and insert culture 注 A:天花板培养法和滤网培养法培养的DFAT细胞CD29阳性表达率差异无统计学意义(P=0.81);B:天花板培养法和滤网培养法培养的DFAT细胞CD90阳性率表达率差异无统计学意义(P=0.98);C:天花板培养法和滤网培养法培养的DFAT细胞CD31阳性表达率差异无统计学意义(P=0.62);D:天花板培养法和滤网培养法培养的DFAT细胞CD45阳性表达率差异无统计学意义(P=0.86) |
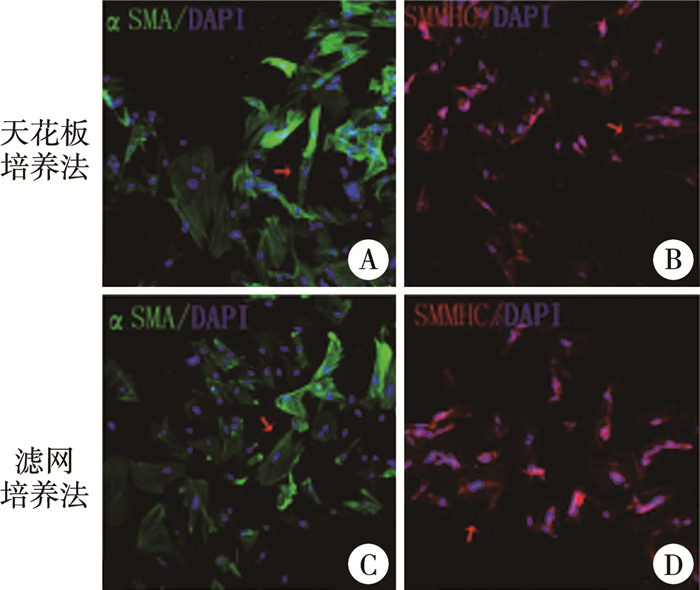
天花板培养法和滤网培养法培养的DFAT细胞经体外诱导后αSMA、SMMHC免疫荧光染色均呈阳性(图 4)。天花板培养法和滤网培养法培养的DFAT细胞经体外诱导后αSMA免疫荧光阳性细胞率分别为(73.67±8.04)%、(73.56±8.44)%,差异无统计学意义(t=0.017,P=0.987)。
|
图 4 天花板培养法和滤网培养法培养的DFAT细胞经体外诱导后免疫荧光结果(Bar=100 μm) Fig.4 Immunofluorescent results of DFAT cells cultured by ceiling culture and insert culture after induction in vitro (Bar=100 μm) 注 A:天花板培养法培养的DFAT细胞经体外诱导后αSMA免疫荧光染色呈阳性;B:天花板培养法培养的DFAT细胞经体外诱导后SMMHC免疫荧光染色呈阳性;C:滤网培养法培养的DFAT细胞经体外诱导后αSMA免疫荧光染色呈阳性;D:滤网培养法培养的DFAT细胞经体外诱导后SMMHC免疫荧光染色呈阳性(红色箭头所示为阳性) |
近年来,干细胞工程研究已取得很多成果,干细胞移植为损伤组织的替代治疗开辟了广泛的应用前景,如心肌损伤、脑损伤等[6-8]。目前研究较多的多能干细胞和成体干细胞主要有骨髓间充质干细胞(bone marrow mesenchymal cells, BM-MSCs)、脂肪干/基质细胞(adipose derived stem/stromal cell, ASCs)和DFAT细胞。BM-MSCs和ASCs具有高度异质性,分离培养后可能含有成纤维细胞、内皮细胞等其他类型细胞。研究表明,经多次传代后,ASCs仍表达多种内皮细胞标志物,而DFAT细胞在第一代就几乎不含其他类型细胞,具有较高的同质性,临床应用安全性更高。其次,DFAT细胞从少量脂肪组织中即可获得,且获取DFAT细胞时不受患者年龄限制,可用于老龄患者[9-13]。因此,目前DFAT细胞已成为大多数学者研究干细胞工程的种子细胞。
DFAT细胞来源于成熟白色脂肪细胞,具有良好的增殖分化潜能和低免疫原性[14]。基于脂肪细胞的漂浮性,Sugihara等[4]提出通过天花板培养法培养DFAT细胞。天花板培养法培养的DFAT细胞能表达多种胚胎干细胞标志物,包括POU同源结构域蛋白(Pit-Oct-Unc class 5 homeobox 1, Oct3/4)、性别决定区Y框蛋白2(sex determining region Y box protein 2, Sox2)、C-myc和同源结构域蛋白(homeobox protein, Nanog)[14]。DFAT细胞在体内可分化为平滑肌细胞,修复小鼠膀胱壁冷冻损伤、改善大鼠漏尿压;可分化为髓核样细胞,促进大鼠椎间盘再生;还可分化为心肌细胞,促进大鼠急性心肌梗死后梗死部位血管生成[8, 15-17]。DFAT细胞可在体内生成巢蛋白(Nestin)和Sox2,促进小鼠脑梗死后功能恢复,修复新生大鼠缺血缺氧性脑损伤,诱导小鼠脊髓损伤后功能恢复[18-20]。此外,DFAT细胞可在小鼠体内分化为脂肪细胞,生成脂肪垫[21]。DFAT细胞在体外可分化为内皮细胞,参与新生血管形成;可分化为成骨细胞,表达Runt相关转录因子2(Runt-related transcription factor 2, Runx2);可分化为软骨细胞,表达Y染色体性别决定区(sex determing region of Y chromosome, SRY)-盒转录因子9(SRY-box transcription factor 9, Sox9);还可分化为骨骼肌细胞,表达成肌分化蛋白(myogenic differentiation, MyoD)等[22-32]。因此,DFAT细胞有望用于修复多种器官及组织的损伤。
Jumabay等[3]提出通过滤网培养法培养DFAT细胞,DFAT细胞可直接从滤网下沉到六孔板底部,脂肪细胞留在滤网上,取出滤网可减少脂肪细胞对DFAT细胞生长的影响。滤网培养法培养的人DFAT细胞能表达外胚层标记Nestin、微管相关蛋白2(microtubule-associated proteins 2, MAP-2),中胚层标记转录因子Nkx2-5(NK2 homeobox 5, Nkx2-5)和转录因子Tbx5(T-box transcription factor 5, Tbx5),内胚层标记甲胎蛋白和GATA结合蛋白6(transcription factor Gata 6, GATA6),多能性标志物Oct3/4、Kruppel样因子4(Krüppel-like factor 4, Klf4)、Sox2和Nanog[6]。滤网培养法培养的人DFAT细胞的多能性标志物的表达量比天花板培养法高3~6倍[3, 6]。另外,在裸鼠畸胎瘤实验中,DFAT细胞可以分化为3个胚层且不形成畸胎瘤,表明滤网培养法培养的DFAT细胞具有多能性和分化潜能[6]。目前国内外只有Jumabay[3]课题组发表过采用滤网培养法培养人和小鼠DFAT细胞,且滤网培养法培养的DFAT细胞分化为平滑肌细胞的潜能也未有报道。因此,我们采用两种方法培养大鼠DFAT细胞,并比较其分化为平滑肌细胞的能力,旨在获得一种更有优势的培养方法。
本研究中两种培养方法培养的DFAT细胞形态均呈成纤维细胞样,梭形、峰谷样排列,与文献中天花板培养法培养的DFAT细胞形态一致[7]。Jumabay等[3]描述的滤网培养法培养的人和小鼠DFAT细胞呈团簇样生长,而本实验在诱导DFAT细胞分化和转染病毒过程中偶见团簇样生长的细胞,猜测可能是物种不同或损伤等因素导致细胞生长形态差异。另外,Jumabay等[3]提出的滤网培养法中,成熟细胞接种至六孔板5 d时取出滤网;而实验中发现5 d时六孔板底部细胞量较少,因此选择在六孔板底部成纤维样细胞量达到约30%时取出滤网。本研究中两种方法培养DFAT细胞生长至首次传代所需的时间没有显著差异,表明两种培养方法时间成本没有差异。然而,天花板培养法培养DFAT细胞生长至首次传代较滤网培养法需要更多的完全培养基,表明天花板培养法经济成本稍高。
研究表明,DFAT细胞表达CD29、CD90、内皮糖蛋白(Endoglin, CD105)、透明质酸受体(hyaluronate receptor, CD44)和5'-核苷酸酶(5'-nucleotidase, CD73)等,不表达CD31、CD45、造血祖细胞抗原(hematopoietic progenitor cell antigen, CD34)和血管细胞粘附分子1(vascular cell adhesion molecule 1, CD106)等[1-2]。CD29和CD90与干细胞的增殖和分化有关,可用来鉴定干细胞[33-35]。CD31在血管内皮细胞中表达,CD45在造血细胞中表达,二者可用来测定DFAT细胞的纯度[36]。因此,本研究中测定DFAT细胞表面抗原标志物CD29、CD90、CD31和CD45的表达量,两种方法培养的DFAT细胞高表达CD29、CD90,均超过95%,提示其与BM-MSCs和ASCs表型相似[37];而Jumabay等[3]报道滤网培养法培养的DFAT细胞CD90阳性率为(2.2±0.1)%。这一差异可能是提取脂肪组织部位、实验室环境及物种不同所致,也可能是进行实验时选择的DFAT细胞代数不同所致。本研究中,两种方法培养的DFAT细胞低表达CD31、CD45,均小于1%,与文献中结果一致,表明两种方法培养的DFAT细胞同质性均很高,为其安全应用于再生工程提供了良好的基础[19]。
本研究中天花板培养法和滤网培养法培养的DFAT细胞经体外诱导后均能分化为平滑肌细胞,表达αSMA和SMMHC,αSMA免疫荧光阳性细胞率分别为(73.67±8.04)%、(73.56±8.44)%,与文献中天花板培养的DFAT细胞分化能力一致[5];表明两种方法培养的DFAT细胞均具有较高的分化为平滑肌细胞的潜能,为其应用于修复平滑肌细胞损伤提供了可能。
本研究中两种培养方法均可获得大量DFAT细胞,但滤网培养法培养过程中采用30~50 uL静置24 h后的乳脂层脂肪细胞接种至六孔板单孔中,无法精确最终的接种密度,因此本文未从节省脂肪组织方面进行比较。有研究曾用肾周脂肪分离培养ASCs,我们也尝试用肾周脂肪分离成熟脂肪细胞,但实验中发现,肾周脂肪中结缔组织较多,加入等体积0.1%的Ⅰ型胶原酶消化组织时,肾周脂肪消化所需时间较腹股沟皮下脂肪长,且获取腹股沟皮下脂肪不需开腹,较肾周脂肪更为方便、安全[38]。
综上所述,天花板培养法和滤网培养法培养DFAT细胞在时间成本、细胞形态、细胞纯度及体外诱导分化为平滑肌细胞的潜能方面均无显著差异,但从经济成本考虑,滤网培养法稍有优势。
利益冲突 所有作者声明不存在利益冲突
作者贡献声明 文献检索为陈晨、刘鑫;实验过程为陈晨、刘鑫、刘舸、范旭;数据分析为陈晨;论文结果撰写为陈晨;论文讨论分析为陈晨、刘鑫、杨屹
| [1] |
Yagi K, Kondo D, Okazaki Y, et al. A novel preadipocyte cell line established from mouse adult mature adipocytes[J]. Biochem Biophys Res Commun, 2004, 321(4): 967-974. DOI:10.1016/j.bbrc.2004.07.055 |
| [2] |
Wei SJ, Zan LS, Hausman GJ, et al. Dedifferentiated adipocyte-derived progeny cells (DFAT cells): potential stem cells of adipose tissue[J]. Adipocyte, 2013, 2(3): 122-127. DOI:10.4161/adip.23784 |
| [3] |
Jumabay M, Abdmaulen R, Ly A, et al. Pluripotent stem cells derived from mouse and human white mature adipocytes[J]. Stem Cells Transl Med, 2014, 3(2): 161-171. DOI:10.5966/sctm.2013-0107 |
| [4] |
Sugihara H, Yonemitsu N, Miyabara S, et al. Primary cultures of unilocular fat cells: characteristics of growth in vitro and changes in differentiation properties[J]. Differentiation, 1986, 31(1): 42-49. DOI:10.1111/j.1432-0436.1986.tb00381.x |
| [5] |
Sakuma T, Matsumoto T, Kano K, et al. Mature, adipocyte derived, dedifferentiated fat cells can differentiate into smooth muscle-like cells and contribute to bladder tissue regeneration[J]. J Urol, 2009, 182(1): 355-365. DOI:10.1016/j.juro.2009.02.103 |
| [6] |
Jumabay M, Boström KI. Dedifferentiated fat cells: a cell source for regenerative medicine[J]. World J Stem Cells, 2015, 7(10): 1202-1214. DOI:10.4252/wjsc.v7.i10.1202 |
| [7] |
Duarte MS, Bueno R, Silva W, et al. Triennial growth and development symposium: dedifferentiated fat cells: potential and perspectives for their use in clinical and animal science purpose[J]. J Anim Sci, 2017, 95(5): 2255-2260. DOI:10.2527/jas.2016.1094 |
| [8] |
von Heimburg D, Lemperle G, Dippe B, et al. Free transplantation of fat autografts expanded by tissue expanders in rats[J]. Br J Plast Surg, 1994, 47(7): 470-476. DOI:10.1016/0007-1226(94)90029-9 |
| [9] |
Kono S, Kazama T, Kano K, et al. Phenotypic and functional properties of feline dedifferentiated fat cells and adipose-derived stem cells[J]. Vet J, 2014, 199(1): 88-96. DOI:10.1016/j.tvjl.2013.10.033 |
| [10] |
黎润光, 邵景范, 魏明发, 等. 小儿骨髓间充质干细胞的培养及多向分化潜能的研究[J]. 临床小儿外科杂志, 2007, 6(2): 25-28. Li RG, Shao JF, Wei MF, et al. Experiment study of biological characteristics and multi-directional differentiation of children's bone marrow stem cells in vitro[J]. J Clin Ped Sur, 2007, 6(2): 25-28. DOI:10.3969/j.issn.1671-6353.2007.02.009 |
| [11] |
Poloni A, Maurizi G, Leoni P, et al. Human dedifferentiated adipocytes show similar properties to bone marrow-derived mesenchymal stem cells[J]. Stem Cells, 2012, 30(5): 965-974. DOI:10.1002/stem.1067 |
| [12] |
Saler M, Caliogna L, Botta L, et al. hASC and DFAT, multipotent stem cells for regenerative medicine: a comparison of their potential differentiation in vitro[J]. Int J Mol Sci, 2017, 18(12): 2699. DOI:10.3390/ijms18122699 |
| [13] |
Nobusue H, Endo T, Kano K. Establishment of a preadipocyte cell line derived from mature adipocytes of GFP transgenic mice and formation of adipose tissue[J]. Cell Tissue Res, 2008, 332(3): 435-446. DOI:10.1007/s00441-008-0593-9 |
| [14] |
Gao Q, Zhao LL, Song ZY, et al. Expression pattern of embryonic stem cell markers in DFAT cells and ADSCs[J]. Mol Biol Rep, 2012, 39(5): 5791-5804. DOI:10.1007/s11033-011-1371-4 |
| [15] |
Obinata D, Matsumoto T, Ikado Y, et al. Transplantation of mature adipocyte-derived dedifferentiated fat (DFAT) cells improves urethral sphincter contractility in a rat model[J]. Int J Urol, 2011, 18(12): 827-834. DOI:10.1111/j.1442-2042.2011.02865.x |
| [16] |
Jumabay M, Matsumoto T, Yokoyama SI, et al. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats[J]. J Mol Cell Cardiol, 2009, 47(5): 565-575. DOI:10.1016/j.yjmcc.2009.08.004 |
| [17] |
Jumabay M, Zhang R, Yao YC, et al. Spontaneously beating cardiomyocytes derived from white mature adipocytes[J]. Cardiovasc Res, 2010, 85(1): 17-27. DOI:10.1093/cvr/cvp267 |
| [18] |
Kakudo T, Kishimoto N, Matsuyama T, et al. Functional recovery by application of human dedifferentiated fat cells on cerebral infarction mice model[J]. Cytotechnology, 2018, 70(3): 949-959. DOI:10.1007/s10616-018-0193-9 |
| [19] |
Mikrogeorgiou A, Sato Y, Kondo T, et al. Dedifferentiated fat cells as a novel source for cell therapy to target neonatal hypoxic-ischemic encephalopathy[J]. Dev Neurosci, 2017, 39(1/4): 273-286. DOI:10.1159/000455836 |
| [20] |
Yamada H, Ito D, Oki Y, et al. Transplantation of mature adipocyte-derived dedifferentiated fat cells promotes locomotor functional recovery by remyelination and glial scar reduction after spinal cord injury in mice[J]. Biochem Biophys Res Commun, 2014, 454(2): 341-346. DOI:10.1016/j.bbrc.2014.10.082 |
| [21] |
Shen JF, Sugawara A, Yamashita J, et al. Dedifferentiated fat cells: an alternative source of adult multipotent cells from the adipose tissues[J]. Int J Oral Sci, 2011, 3(3): 117-124. DOI:10.4248/IJOS11044 |
| [22] |
Jumabay M, Abdmaulen R, Urs S, et al. Endothelial differentiation in multipotent cells derived from mouse and human white mature adipocytes[J]. J Mol Cell Cardiol, 2012, 53(6): 790-800. DOI:10.1016/j.yjmcc.2012.09.005 |
| [23] |
Suzuki D, Akita D, Tsurumachi N, et al. Transplantation of mature adipocyte-derived dedifferentiated fat cells into three-wall defects in the rat periodontium induces tissue regeneration[J]. J Oral Sci, 2017, 59(4): 611-620. DOI:10.2334/josnusd.16-0878 |
| [24] |
Kishimoto N, Momota Y, Hashimoto Y, et al. Dedifferentiated fat cells differentiate into osteoblasts in Titanium fiber mesh[J]. Cytotechnology, 2013, 65(1): 15-22. DOI:10.1007/s10616-012-9456-z |
| [25] |
Kaida K, Honda Y, Hashimoto Y, et al. Application of green tea catechin for inducing the osteogenic differentiation of human dedifferentiated fat cells in vitro[J]. Int J Mol Sci, 2015, 16(12): 27988-28000. DOI:10.3390/ijms161226081 |
| [26] |
Kishimoto N, Momota Y, Hashimoto Y, et al. The osteoblastic differentiation ability of human dedifferentiated fat cells is higher than that of adipose stem cells from the buccal fat pad[J]. Clin Oral Investig, 2014, 18(8): 1893-1901. DOI:10.1007/s00784-013-1166-1 |
| [27] |
Hao W, Jiang CQ, Jiang M, et al. Osteogenic potency of dedifferentiated fat cells isolated from elderly people with osteoporosis[J]. Exp Ther Med, 2017, 14(1): 43-50. DOI:10.3892/etm.2017.4465 |
| [28] |
Shimizu M, Matsumoto T, Kikuta S, et al. Transplantation of dedifferentiated fat cell-derived micromass pellets contributed to cartilage repair in the rat osteochondral defect model[J]. J Orthop Sci, 2018, 23(4): 688-696. DOI:10.1016/j.jos.2018.03.001 |
| [29] |
Sakamoto F, Hashimoto Y, Kishimoto N, et al. The utility of human dedifferentiated fat cells in bone tissue engineering in vitro[J]. Cytotechnology, 2015, 67(1): 75-84. DOI:10.1007/s10616-013-9659-y |
| [30] |
Okita N, Honda Y, Kishimoto N, et al. Supplementation of Strontium to a chondrogenic medium promotes chondrogenic differentiation of human dedifferentiated fat cells[J]. Tissue Eng Part A, 2015, 21(9/10): 1695-1704. DOI:10.1089/ten.TEA.2014.0282 |
| [31] |
Nakayama E, Matsumoto T, Kazama T, et al. Transplantation of dedifferentiation fat cells promotes intervertebral disc regeneration in a rat intervertebral disc degeneration model[J]. Biochem Biophys Res Commun, 2017, 493(2): 1004-1009. DOI:10.1016/j.bbrc.2017.09.101 |
| [32] |
Kazama T, Fujie M, Endo T, et al. Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro[J]. Biochem Biophys Res Commun, 2008, 377(3): 780-785. DOI:10.1016/j.bbrc.2008.10.046 |
| [33] |
He Q, Ye ZY, Zhou Y, et al. Comparative study of mesenchymal stem cells from rat bone marrow and adipose tissue[J]. Turk J Biol, 2018, 42: 477-489. DOI:10.3906/biy-1802-52 |
| [34] |
Kisselbach L, Merges M, Bossie A, et al. CD90 expression on human primary cells and elimination of contaminating fibroblasts from cell cultures[J]. Cytotechnology, 2009, 59(1): 31-44. DOI:10.1007/s10616-009-9190-3 |
| [35] |
Oswald J, Boxberger S, Jørgensen B, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro[J]. Stem Cells, 2004, 22(3): 377-384. DOI:10.1634/stemcells.22-3-377 |
| [36] |
Cannella V, Piccione G, Altomare R, et al. Differentiation and characterization of rat adipose tissue mesenchymal stem cells into endothelial-like cells[J]. Anat Histol Embryol, 2018, 47(1): 11-20. DOI:10.1111/ahe.12318 |
| [37] |
Matsumoto T, Kano K, Kondo D, et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential[J]. J Cell Physiol, 2008, 215(1): 210-222. DOI:10.1002/jcp.21304 |
| [38] |
Baer PC, Koch B, Hickmann E, et al. Isolation, characterization, differentiation and immunomodulatory capacity of mesenchymal stromal/stem cells from human perirenal adipose tissue[J]. Cells, 2019, 8(11): 0. DOI:10.3390/cells8111346 |
 2023, Vol. 22
2023, Vol. 22


