儿童腱鞘巨细胞瘤(giant cell tumor of tendon sheath, GCTTS)是一种罕见的良性病变,生长周期从几个月到几年不等,好发于手足部位的腱鞘、关节滑膜及囊膜,高发年龄为30~40岁,女性多见于男性,在10岁以下儿童中极为罕见[1-6]。据有关文献报道,10岁及以下儿童GCTTS发病率约为14.3/10万[7]。目前国内外有许多关于成人GCTTS的报道,而关于儿童GCTTS的报道多以个案为主,Gholve等[8]在2007年报道了29例GCTTS,但是该系列设定的儿童标准为 < 18岁,年龄范围较大。因此,本研究纳入既往文献中资料较齐全的29例GCTTS患者,结合本院2例发生于手部的GCTTS患者,总结该病的发病机制、临床表现及治疗方法,以提高临床医生对GCTTS的认识。
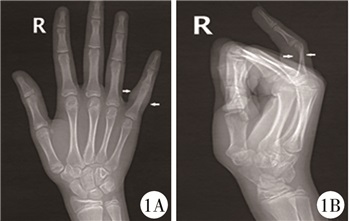
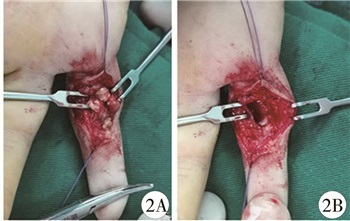
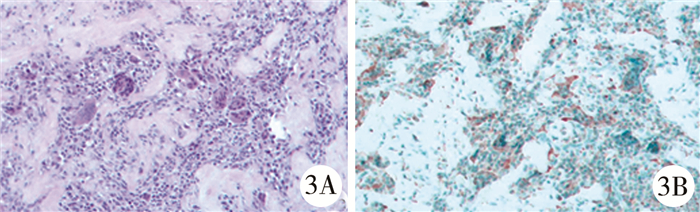
材料与方法 一、临床资料病例1:患者女,9岁9个月,因“右手小指皮下包块1年余”于2019年8月11日入院,专科体格检查:右手小指根部肿胀,皮肤色泽正常,无破溃及出血,皮温正常,无触痛,右手小指近端指间关节屈曲受限。X线检查提示右手小指近节指骨偏远端略细,近端软组织肿胀(图 1)。B超检查提示右手小指近端皮下软组织层普遍性增厚伴其内低回声小结节。患者血常规、大便常规及传染病四项未见明显异常, 于2019年8月13日行皮下肿瘤切除术。术中见肿物呈淡黄色,葡萄状,并与周围肌腱粘连,肿物基底部较宽,约1 cm×1 cm附着于近节指骨远端,指骨远端呈扁平状(图 2)。肿物病理切面所见:组织2枚,共计2.2 cm×1.5 cm×1 cm, 切面呈现灰白色、实性、质中。组织学特征:肿瘤组织由多核巨细胞、单核样细胞及少量泡沫样细胞构成,可见核沟,伴间质胶原化。免疫组化结果提示:CK(-), LCA(-), Desmin(-), SMA(-), CD34(脉管+),Vimentin(+),S-100(-), CD68(+), ALK(-), EMA(-), Ki-67(密集处约15%+)(图 3)。病理诊断为GCTTS。随访至出院后8个月,全身情况好,饮食及大小便均无异常,右手小指各关节活动可。
|
图 1 GCTTS患者(病例1)术前X线平片 注 右手小指近节指骨形态欠佳,偏远端略细,小指近端软组织肿胀 Fig.1 Preoperative radiographic film of GCTTS(case No.1) The morphology of proximal phalanx of right little finger was poor with a slightly thinner distal end |
|
图 2 GCTTS患者(病例1)术中所见 注 肿物呈淡黄色,葡萄状,并与周围肌腱粘连,见肿物基底部较宽,约1 cm×1 cm附着于近节指骨远端,指骨远端被肿物压扁 Fig.2 Intraoperative findings of GCTTS(case No.1) Tumor was yellowish, grape-like and adherent to the surrounding tendons.The base of tumor was relatively wide, around 1 1 square millimeter cm attached to the distal end of proximal phalanx and distal end of phalanx compressed by tumor |
|
图 3 GCTTS患者(病例1)病理检查结果 注 A: 腱鞘巨细胞瘤HE染色(×20);B: 免疫组化标记CD68阳性(×20) Fig.3 Pathological manifestations of GCTTS(case No.1) A: HE routine staining for GCTTS(×20); B: Immunohistochemical marker CD68 positive(×20) |
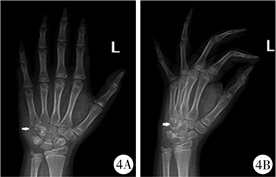
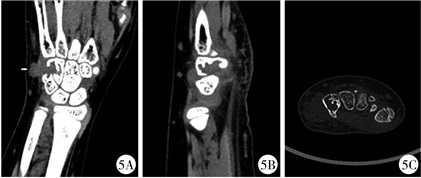
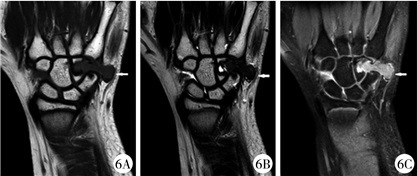
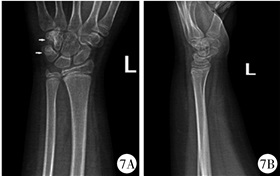
病例2:患者女,8岁11个月,因“发现左腕部包块6 d”于2019年6月3日入院,专科体格检查:左手腕部皮肤色泽正常,无破溃及出血,皮温正常,小鱼际处可扪及约1 cm×1 cm大小包块,质韧,无触痛,不可推动,左腕关节活动无明显受限。X线检查提示左腕骨外侧局部软组织增厚,左侧三角骨及钩骨局部软组织密度减低,见类圆形透亮影(图 4)。B超检查提示左手背至腕部软组织内低回声区。CT检查提示左腕关节三角骨、钩骨可见骨质破坏,局部骨密度减低,周围软组织肿胀(图 5);MRI检查提示左腕三角骨及钩骨内异常信号,并向骨外软组织内突起,周围软组织内见水肿信号(图 6)。患者血常规、大便常规及传染病四项未见明显异常,肿瘤四项检查未见异常。于2019年6月5日行左腕部骨肿瘤切除术+同种异体骨植骨术。术中见黄色类脂肪组织,三角骨及钩骨骨质破坏。肿物病理切面所见:灰黄色碎组织为1.5 cm×1.5 cm×0.5 cm大小, 质软。病理检查表现类似于病例1。病理诊断为GCTTS。随访至患者出院后10个月,全身情况好,饮食及大小便均无异常,左腕关节活动好,X线平片提示左钩骨及三角骨密度增高(图 7)。
|
图 4 GCTTS患者(病例2)术前X线平片 注 左腕骨外侧局部软组织增厚,左侧三角骨及钩骨局部软组织密度减低,见类圆形透亮影 Fig.4 Preoperative radiography of GCTTS(case No.2) Local soft tissue of lateral side of left carpal bone became thickened, and the density of left triangular bone and uncarpal bone was reduced, showing a quasi-circular and bright shadow |
|
图 5 GCTTS患者(病例2)术前CT检查 Fig.5 Preoperative manifestations of GCTTS(case No.2) a: coronal plane; b: sagittal plane; c: cross section; triangular bone and tamate bone of left wrist joint showed bone destruction, local bone density decreased with a swelling of the surrounding soft tissue |
|
图 6 GCTTS患者(病例2)术前MRI检查 注 7A: T1序列; 7B: T2序列; 7C: PDW序列; 左腕三角骨及钩骨内见不规则形态稍长T1信号稍长T2信号,PDW压脂序列为高信号,并向骨外软组织内突出,周围软组织内见水肿信号 Fig.6 Preoperative MRI findings of GCTTS(case No.2) a: T1 sequence; b: T2 sequence; c: PDW sequence; slightly longer T1 and longer T2 signals were detected in left carpal triangle bone and hamate bone.PDW sequence was hyperintense and protruded into extraosseous soft tissue and edema was observed in the surrounding soft tissue. |
|
图 7 GCTTS患者(病例2)术后10个月X线平片 注 三角骨及钩骨密度增高 Fig.7 Radiographic follow-ups of GCTTS(case No.2) at Month 10 post-operation Note: The densities of triangular and hook bones increased. |
通过检索万方、维普、CNKI、PubMed、Medline数据库, 检索关键词:腱鞘巨细胞瘤(giant cell tumor of tendon sheath);色素沉着绒毛结节性滑膜炎(pigmen ted villonodular synovitis)。检索截止日期:2020年7月。剔除成人、大龄儿童病例以及无法获得全文资料的儿童病例,共检索到年龄不超过10岁的儿童GCTTS患者29例(表 1)。
| 表 1 截止2020年7月文献报道的年龄不超过10岁GCTTS患者一般资料 Table 1 General profiles of GCTTS children aged under 10 years in literature prior to July 2020 |
|
|
首发症状:无痛性包块13例(41%),疼痛肿胀5例(16%),单纯肿胀4例(13%),单纯疼痛2例(6%),疼痛跛行2例(6%),疼痛包块1例(3%),疼痛发热1例(3%),疼痛伴活动受限1例(3%),肿胀伴活动受限1例(3%),肿胀伴跛行1例(3%),单纯活动受限1例(3%)。
二、影像学表现6例行B超检查,5例有低回声包块信号,1例表现以积液为主;26例行X线平片检查,17例无阳性表现,4例为溶骨性骨破坏,3例为软组织肿块,1例为类圆形透亮影,1例为钙化结节堆积;2例行CT检查,均表现为骨质破坏伴软组织肿胀;24例行MRI检查,其中19例表现为T1及T2序列上低信号表现,2例表现为T1序列上与周围肌肉等信号,T2序列高信号,给予钆喷酸葡胺(gadolinium diethylenetrianmine pentaacetic acid, Gd-DTPA)对比剂后病变呈不均匀强化,其中11例提示肿块信号,6例提滑膜增厚,3例可见滑膜增厚及关节积液,2例骨破坏。
三、手术及预后共31例中,25例行开放手术,5例行关节镜治疗,1例予开放手术+关节镜联合治疗。随访时间1~156个月(平均随访38个月),明确术后复发1例。
讨论GCTTS最早由Chassaignac于1852年报道。1941年Jaffe等[9]将发生于腱鞘滑膜、滑囊和关节滑膜的病变归于一类。根据目前世界卫生组织的分类, GCTTS包括局限型和弥漫型,局限型主要发生于小关节,尤其是指趾关节,而弥漫型多发生于较年轻的患者, 并可侵袭大关节如膝关节、踝关节、髋关节等[1]。本院收治的2例均属于局限型。GCTTS的病因尚不清楚, 可能与既往创伤、关节内出血或慢性炎症有关[2]。目前局部关节的脂质代谢紊乱也被假定为其原因之一[11]。也有研究认为与染色体异常有关,如第5号、第7号染色体三体畸形[25]。既往文献提示儿童GCTTS的发病部位无上下肢偏向性,病变通常表现为逐渐增大的肿块[8]。相对于成人,儿童GCTTS有侵犯骺板导致生长畸形的可能,本研究有1例侵及股骨头骺板导致双下肢不等长[14]。
典型GCTTS病理表现由滑膜样单核细胞组成,伴数量不等的多核巨细胞、泡沫细胞及含铁血黄素[26], 免疫组化提示CD68阳性[27]。本院收治的2例病理检查均可见滑膜样单核细胞组成,伴数量不等的多核巨细胞、泡沫细胞,同时免疫组化均提示CD68阳性,故诊断为GCTTS。
本研究患者男女比例为14:17,病变可见于膝关节、手指、髋关节、肘关节及足踝部等处,以膝关节为主,共计12例(38.7%)。Somerhausen等[28]报道的50例GCTTS中,年龄范围为4~76岁(平均41.1岁),男女比例为22:28,病变主要见于腕部,占18%,膝关节占16%,大腿和足部占12%,手指占10%;Batra等[29]报道的48例GCTTS患者中,年龄范围为11~60岁(平均23岁),男女比例约为1:2,病变主要位于手指(36例,75%)和足趾(7例,14%)。本研究与以上两个大样本研究比较,性别分布均以女性为主,病变发生位置都较为广泛,但位置分布稍有差异,说明了GCTTS可发生于全身,且儿童与成人的GCTTS好发部位有一定差异。笔者认为,病变部位分布的差异会造成主要症状的不同,本组患者临床症状以无痛性包块为主(38.7%)。Capellen等[30]研究中,主要症状为疼痛(54%)和肿胀(40%)。本研究中手足部等浅表部位占比达41.9%,Capellen研究中以膝关节处病变为主(55%),膝关节作为人体主要的承重结构且具有较大的关节腔,其相对于手足部等浅表位置,不易发现包块,更易以疼痛肿胀为首发症状。以上充分说明了GCTTS症状多,分布广,临床表现无明显特异性的特点。
Wang等[31]认为超声可以判断肿瘤与相邻组织的关系,提示超声可以作为诊断GCTTS的首选方法,尽管本研究中行B超检查的患者数目不多,但结果都提示小结节或低回声包块等阳性表现,虽然无法定性,但已满足定位要求。本组X线检查阳性率为34.6%,Baroni等[12]研究中X线检查阳性率为33%,Gholve等[8]报道为48%,阳性率都不超过50%。此外,X线对于GCTTS患者骨破坏检出率仅为10% ~20%[29]。故笔者认为,术前X线对于GCTTS常规诊断价值有限,且只有当肿块跨越关节生长同时合并邻近骨质出现压迫性缺损时才能有较高的诊断价值[32]。Chiari等[33]研究认为MRI检查提示为典型的含铁血黄素沉积病变,导致T1加权像呈现低至中等信号强度,T2加权像呈低信号强度,本研究中确定行MRI检查的患者相对较多,且主要表现为T1和T2序列上呈现低信号,这与本研究结果相符。GCTTS本身发生骨质破坏并不多,发生率为9% ~25%[8],且真正的骨内GCTTS报道也相当少,病例2属于骨内GCTTS且CT检查提示存在骨质破坏,说明CT对于骨皮质的破坏以及结构复杂、重叠较多病灶的显示有一定优势。但笔者发现既往成人GCTTS报道中[34],CT检查应用不多,推测是因为骨内GCTTS少见,且CT检查对于软组织分辨率不如MRI检查。笔者认为在GCTTS的影像学检查中,超声作为初步诊断可以很好地判断肿瘤与相邻组织的关系,X线及CT检查不是GCTTS常规选择,MRI检查对GCTTS的显示更敏感,在软组织分辨率较高的同时,还能从任何方位将GCTTS的形态、位置、信号强度、毗邻关系及侵犯范围等成像显示,对于GCTTS的手术治疗更具指导意义,且有利于减少创伤及完整切除肿瘤。除常规影像学检查之外,细针穿刺细胞学(fine needle aspiration cytology, FNAC)作为一种快速、简便、高效的诊断方法,也已经应用到了临床[35]。
GCTTS极少发生于儿童, 由于临床和影像学资料特异性不强,临床医生容易忽略此病[27]。主要鉴别诊断包括:滑膜肉瘤、腱鞘纤维瘤、异物肉芽肿、血管球瘤及腱鞘黄色瘤等。滑膜肉瘤好发于四肢大关节旁,多有骨膜反应等恶性表现;腱鞘纤维瘤免疫组化检查对actin和Vimentin呈阳性反应而不表达CD68;异物肉芽肿中多核巨细胞及组织细胞相对于纤维母细胞核上皮样细胞来说数量较少,多可发现异物;血管球瘤由血管的动静脉吻合产生,表面呈蓝色或深红色, 多发于指(趾)末端,腱鞘黄色瘤病变常可见胆固醇裂隙,伴有血脂升高,该特点在GCTTS中不多见[36, 37]。
GCTTS的治疗首选是手术治疗。本研究31例患者中25例行开放手术,5例行关节镜治疗,1例开放手术+关节镜联合治疗。手术治疗时必须注意保护肌腱,通常由于存在假包膜,应尽可能对肿瘤进行整块切除,同时应该对周围所有组织的卫星病变及与之连接的病变进行切除[38]。对伴有骨破坏的GCTTS病变行刮除术时,一定要注意保护骨骺,避免造成医源性生长发育畸形。本院收治的2例患者皆于术中仔细剥离肿物,分离与其粘连肌腱,处理囊壁,实施了规范化手术治疗。目前开放手术和关节镜下滑膜次全切除术是应用最广的手术方式。关于两者孰优孰劣,尚无统一定论。部分学者推崇关节镜手术治疗局限型GCTTS,因为相较于开放手术,关节镜手术在减少并发症的同时,也不会影响手术疗效[39]。而另一些学者则更倾向于开放手术,因为他们认为关节镜手术后复发率较高且手术视野污染风险较大[12]。笔者认为目前治疗的主要方式仍是开放性手术治疗,关节镜治疗不失为一种选择。巨噬细胞集落刺激因子(colony stimulating factor, CSF1)是一种细胞因子/造血生长因子,CSF1与其受体CSF1R形成的信号通路与GCTTS的发病机制有一定关系。斯坦福大学的研究人员曾在GCTTS的大多数细胞中检测到CSFR1受体的高水平表达,同时考虑到CSFR1是酪氨酸受体,既往已有酪氨酸激酶受体抑制剂(如伊马替尼)应用于治疗GCTTS的报道[10]。最近, 新的CSFR1阻滞剂已被开发出来并正在临床试验中[19]。英夫利昔单抗已成功应用于髋部GCTTS的治疗, 可以逐渐改善临床症状,减轻疼痛,从而延缓或避免骨和关节的损坏,故抗肿瘤坏死因子(Tumor necrosis factor,TNF)抗体也被认为是一种有效的补充治疗[40]。但目前并未大规模应用于临床,国内外关于此类药物治疗尚缺乏大样本系统研究,这或许是本病未来需要研究的方向。
复发是GCTTS术后的一个主要问题,本研究31例随访时间为1~156个月(平均随访时间为38个月),明确术后复发1例,考虑到相关文献报道成人患者10% ~44%的复发率[8],笔者认为相比于成人,年龄较小的GCTTS患者缺乏邻近的关节改变(包括关节炎)等危险因素,且好发于手指等浅表部位,皆以肿块为首发症状,有利于早期发现,早期治疗,故这些因素可能有助于减少复发。值得一提的是,骨破坏的存在已被报道为GCTTS复发的一个重要因素[31],本研究中病例2存在骨破坏,需要长期随访。
总之,儿童GCTTS非常少见,临床症状无特异性, X线及CT检查对于GCTTS诊断价值不大,术前超声检查及MRI检查有助于初步诊断GCTTS,但明确诊断仍需要结合组织病理学检查,首次规范化手术是治疗成败的关键,复发和恶变使得GCTTS患者长期随访是必不可少的。
| [1] |
Yun SJ, Hwang SY, Jin W, et al. Intramuscular diffuse-type tenosynovial giant cell tumor of the deltoid muscle in a child[J]. Skeletal Radiol, 2014, 43(8): 1179-1183. DOI:10.1007/s00256-014-1854-1 |
| [2] |
Lee DH, Lim SY. A rare giant cell tumor of the tendon sheath of the dorsal hand in a child[J]. Arch Plast Surg, 2014, 41(5): 605-606. DOI:10.5999/aps.2014.41.5.605 |
| [3] |
Ansari SMM, Tambe SA, Nayak CS. Giant cell tumor of tendon sheath in children[J]. Indian J Dermatol Venereol Leprol, 2019, 85(5): 496-498. DOI:10.4103/ijdvl.IJDVL_471_18 |
| [4] |
Mukhopadhyay K, Smith M, Hughes PM. Multifocal PVNS in a child-followed over 25 years[J]. Skeletal Radiol, 2006, 35(7): 539-542. DOI:10.1007/s00256-005-0013-0 |
| [5] |
Tsujino S, Matsumoto S, Ae K. Tenosynovial giant cell tumour of the hand in children under 10 years of age[J]. J Hand Surg Eur Vol, 2018, 43(3): 335-337. DOI:10.1177/1753193417739518 |
| [6] |
Mills JK, Wheeler L, Oishi SN. A painful finger mass in an 8-year-old child[J]. JAAPA, 2015, 28(9): 20-21. DOI:10.1097/01.jaa.0000470439.14941.d1 |
| [7] |
Mastboom MJL, Verspoor FGM, Verschoor AJ, et al. Higher incidence rates than previously known in tenosynovial giant cell tumors.A nationwide study in The Netherlands[J]. Acta Orthop, 2017, 88(1): 1-7. DOI:10.1080/17453674.2017.1361126 |
| [8] |
Gholve PA, Hosalkar HS, Kreiger PA, et al. Giant cell tumor of tendon sheath: largest single series in children[J]. J Pediatr Orthop, 2007, 27(1): 67-74. DOI:10.1097/01.bpo.0000242380.95348.8b |
| [9] |
Ueno T, Ansai SI, Omi T, et al. A child with giant cell tumor of tendon sheath[J]. Dermatol Online J, 2011, 17(5): 9. |
| [10] |
Neubauer P, Weber K, Miller NH, et al. Pigmented villonodular synovitis in children: a report of six cases and review of the literature[J]. Iowa Orthop J, 2007, 27: 90-94. |
| [11] |
于丹, 王千, 刘鸿坚, 等. 小儿腱鞘巨细胞瘤1例[J]. 临床小儿外科杂志, 2010, 9(4): 299. Yu D, Wang Q, Liu HJ, et al. A case of giant cell tumor of tendon sheath in a child[J]. J Clin Ped Sur, 2010, 9(4): 299. DOI:10.3969/j.issn.1671-6353.2010.04.020 |
| [12] |
Baroni E, Russo BD, Masquijo JJ, et al. Pigmented villonodular synovitis of the knee in skeletally immature patients[J]. J Child Orthop, 2010, 4(2): 123-127. DOI:10.1007/s11832-009-0236-z |
| [13] |
Sekiya H, Ozawa H, Sugimoto N, et al. Pigmented villonodular synovitis of the elbow in a 6-year-old girl: a case report[J]. J Orthop Surg (Hong Kong), 2007, 15(1): 106-108. DOI:10.1177/230949900701500124 |
| [14] |
Higuchi C, Ohno I, Yoshikawa H. Hip joint pigmented villonodular synovitis in a young girl: a case report[J]. J Pediatr Orthop B, 2012, 21(4): 335-338. DOI:10.1097/BPB.0b013e3283524bdf |
| [15] |
Balakrishnan V, Dunn AL. A case of pigmented villonodular synovitis in conjunction with a platelet release defect in a paediatric patient[J]. Haemophilia, 2013, 19(2): e89-e90. DOI:10.1111/hae.12062 |
| [16] |
Pandey S, Pandey AK. Pigmented villonodular synovitis with bone involvement[J]. Arch Orthop Trauma Surg, 1981, 98(3): 217-223. DOI:10.1007/BF00632980 |
| [17] |
Aghasi MK, Robinson D, Reif RM, et al. Pigmented villonodular synovitis of the talus in a child[J]. Foot & ankle, 1988, 9(3): 139-142. |
| [18] |
Givon U, Ganel A, Heim M. Pigmented villonodular synovitis[J]. Rev Med Suisse, 1991, 66(12): 1449-1450. |
| [19] |
Saadat E, Vargas SO, Anderson M, et al. Pediatric intra-articular localized tenosynovial giant cell tumor presenting as an acutely irritable hip: a case report[J]. JBJS Case Connect, 2016, 6(3): e60. DOI:10.2106/JBJS.CC.15.00291 |
| [20] |
Hwang JS, Fitzhugh VA, Gibson PD, et al. Multiple giant cell tumors of tendon sheath found within a single digit of a 9-year-old[J]. Case Rep Orthop, 2016, 2016: 1834740. DOI:10.1155/2016/1834740 |
| [21] |
Jha SC, Nishisho T, Matsuura T, et al. Pigmented villonodular synovitis of the knee joint in a 5-year-old girl treated with combined open and arthroscopic surgery: a case report[J]. J Pediatr Orthop B, 2016, 25(6): 564-569. DOI:10.1097/BPB.0000000000000293 |
| [22] |
Saulsbury FT. Pigmented villonodular synovitis of the knee in a 9-year-old child[J]. South Med J, 2004, 97(1): 80-83. DOI:10.1097/01.SMJ.0000104842.97001.88 |
| [23] |
Sébastien Ottaviani, Ayral X, Dougados M, et al. Pigmented villonodular synovitis: a retrospective single-center study of 122 cases and review of the literature[J]. Semin Arthritis Rheum, 2011, 40(6): 539-546. DOI:10.1016/j.semarthrit.2010.07.005 |
| [24] |
Zheng S, Lee PY, Huang YK, et al. Giant cell tumor of tendon sheath and tendinopathy as early features of early onset sarcoidosis[J]. Front Pediatr, 2019, 7: 480. DOI:10.3389/fped.2019.00480 |
| [25] |
Artur, Fałek, Joanna, et al. Pigmented villonodular synovitis[J]. Folia medica Cracoviensia, 2019, 58(4): 93-104. |
| [26] |
张志国, 吴文海, 李舒琪, 等. 腱鞘巨细胞瘤的临床病理学及影像学分析[J]. 中国医疗设备, 2015, 30(2): 44-48. Zhang ZG, Wu WH, Li SQ, et al. Analysis of clinicopathological features and imaging findings of patients with GCTTS[J]. China Medical Devices, 2015, 30(2): 44-48. DOI:10.3969/j.issn.1674-1633.2015.02.011 |
| [27] |
李翀, 李海. 腱鞘巨细胞瘤40例临床病理分析[J]. 临床和实验医学杂志, 2009, 8(8): 122-123. Li C, Li H. Clinicopathologic analysis of giant cell tumor of tendon sheath: a report of 40 cases[J]. Am J Surg Pathol, 2009, 8(8): 122-123. DOI:10.3969/j.issn.1671-4695.2009.08.067 |
| [28] |
Somerhausen NS, Fletcher CD. Diffuse-type giant cell tumor: clinicopathologic and immunohistochemical analysis of 50 cases with extraarticular disease[J]. Am J Surg Pathol, 2000, 24(4): 479-492. DOI:10.1097/00000478-200004000-00001 |
| [29] |
Batra VV, Jain S, Singh DK, et al. Cytomorphologic spectrum of giant cell tumor of tendon sheath[J]. Acta Cytol, 2008, 52(2): 152-158. DOI:10.1159/000325473 |
| [30] |
Capellen CF, Tiling R, Klein A, et al. Lowering the recurrence rate in pigmented villonodular synovitis: A series of 120 resections[J]. Rheumatology (Oxford), 2018, 57(8): 1448-1452. DOI:10.1093/rheumatology/key133 |
| [31] |
Wang YX, Tang J, Luo YK. The value of sonography in diagnosing giant cell tumors of the tendon sheath[J]. J Ultrasound Med, 2007, 26(10): 1333-1340. DOI:10.7863/jum.2007.26.10.1333 |
| [32] |
欧建宏, 黄耀华. 手足部局限型腱鞘巨细胞瘤X线及MRI诊断[J]. 中国实用医药, 2015, 10(23): 91-92. Ou JH, Huang YH. Radiographic and MRI diagnosis of localized giant cell tumor of tendon sheath in hand and foot[J]. China Prac Med, 2015, 10(23): 91-92. DOI:10.14163/j.cnki.11-5547/r.2015.23.055 |
| [33] |
Chiari C, Pirich C, Brannath W, et al. What affects the recurrence and clinical outcome of pigmented villonodular synovitis?[J]. Clin Orthop Relat Res, 2006, 450(450): 172-178. DOI:10.1097/01.blo.0000224051.01873.fb |
| [34] |
Di Grazias, Succi G, Fraggetta F, et al. Giant cell tumor of tendon sheath: study of 64 cases and review of literature[J]. G Chir, 2013, 34(5-6): 149-152. DOI:10.11138/gchir/2013.34.5.149 |
| [35] |
Aditi, Goyal, Priya, et al. Role of FNAC in diagnosing lesions of hand and wrist[J]. Diagn Cytopathol, 2018, 46(10): 853-858. DOI:10.1002/dc.24050 |
| [36] |
袁绍辉, 曾文军. 腱鞘巨细胞瘤1例[J]. 皮肤性病诊疗学杂志, 2014, 21(6): 487-488. Yuan SH, Zeng WJ. One case of giant cell tumor of tendon sheath[J]. J Diagn Ther Dermato-Venereol, 2014, 21(6): 487-488. DOI:10.3969/j.issn.1674-8468.2014.06.021 |
| [37] |
任建璋. 手指腱鞘巨细胞瘤一例报告[J]. 现代实用医学, 2013, 25(10): 1197. Ren JZ. Giant cell tumor of tendon sheath of finger: a case report[J]. Modern Practical Medicine, 2013, 25(10): 1197. DOI:10.3969/j.issn.1671-0800.2013.10.070 |
| [38] |
Adams EL, Yoder EM, Kasdan ML. Giant cell tumor of the tendon sheath: experience with 65 cases[J]. Eplasty, 2012, 12: e50. |
| [39] |
Linney LS, Al-Hassani F, Pikturnaite J, et al. Tenosynovial giant cell tumours of the hand: a multicentre case-control study[J]. J Plast Reconstr Aesthet Surg, 2019, 72(6): 918-923. DOI:10.1016/j.bjps.2019.01.021 |
| [40] |
Flemming DJ, Walker EA. From the archives of the afip pigmented villonodular synovitis[J]. Health San Francisco, 2008, 28(5): 1493-1518. DOI:10.1148/rg.285085134 |
 2021, Vol. 20
2021, Vol. 20


