2. 国家儿童医学中心, 首都医科大学附属北京儿童医院新生儿外科(北京市, 100045);
3. 国家儿童医学中心, 首都医科大学附属北京儿童医院普通外科(北京市, 100045);
4. 国家儿童医学中心, 首都医科大学附属北京儿童医院病理科(北京市, 100045)
2. Department of Neonatal Surgery, National Center of Children's Health, Beijing Children's Hospital, Capital Medical University, Beijing 100045, China;
3. Department of General Surgery, National Center of Children's Health, Beijing Children's Hospital, Capital Medical University, Beijing 100045, China;
4. Department of Pathology, National Center of Children's Health, Beijing Children's Hospital, Capital Medical University, Beijing 100045, China
婴儿型纤维肉瘤(infantile fibrosarcoma, IFS)是一种罕见的恶性软组织肿瘤,在儿童肿瘤中占比 < 1%,多发病于1岁以内,又称先天性纤维肉瘤[1]。与成人纤维肉瘤不同,IFS恶性程度较低,90%以上的患者可以治愈[2]。大多数肿瘤位于四肢和躯干,表现为无痛性肿块,极少侵犯肠道。本文报道国内首例肠道婴儿型纤维肉瘤,并复习相关文献,探讨其临床特征、诊治策略、病理特点及预后。
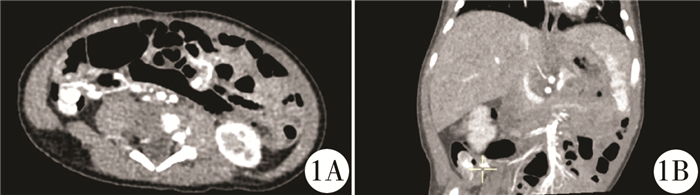
材料与方法 一、临床资料患者,男,1个月13天,因便血3周入院。3周前无明显诱因出现少量红色血便,进食后加重,当地医院予禁食、禁水、胃肠减压,采用维生素K1止血治疗,患者大便转为暗红色。入院查体:口唇稍白,精神反应可,直肠指诊可见暗红色血便。实验室检查:血红蛋白82 g/L(正常值>110 g/L),神经元特异性烯醇化酶39.9 ng/ml(正常值5~15 ng/ml),其它检查结果无明显异常。腹部B超提示右下腹部异常肿胀肠袢,肠腔内多发息肉样病变,肠壁及息肉血供丰富。因怀疑肿瘤病变,行腹部增强CT提示右下腹部异常强化团块影,大小约18.5 mm×8.5 mm×8.9 mm,肠系膜下动脉分支供血,部分深入肠腔内,肿块局部与肠壁分界不清(图 1)。入院诊断:①消化道出血;②消化道肿物性质待查;③中度贫血。入院后予禁食、禁水、胃肠减压等保守治疗,完善检查,拟行腹腔镜探查术。
|
图 1 婴儿型纤维肉瘤患者术前腹部增强CT图 Fig.1 Abdomen contrast-enhanced CT 注 右下腹异常强化包块影,部分伸入肠腔内,包块大小约18.5 mm×8.5 mm×8.9 mm,肠系膜下动脉分支供血 |
静脉吸入复合麻醉后取仰卧位,建立气腹并放置Trocar及腹腔镜器械。腹腔镜下先探查右下腹回盲部区域,回肠末端、回盲部、升结肠等常见出血性疾病(梅克尔憩室、肠重复畸形、血管瘤)好发部位均未见异常;转而探查左下腹区域,可见乙状结肠和降结肠交界处肠管增粗,触及肠腔内包块,腹腔内未见其他病灶及肿大淋巴结。扩大脐部切口将其提出,打开肠壁可见息肉样改变,较大者为1.0 cm×0.7 cm×0.6 cm,周围肠壁存在腺瘤样改变,完整切除病变所在肠管(约6 cm)。端端吻合结肠并缝合系膜裂孔,查腹腔内无活动性出血,拔除Trocar并关闭Trocar口。
三、文献检索通过PubMed和万方数据库,检索国内外肠道婴儿型纤维肉瘤的相关文献,因肠道病变位置不一,检索词为“infantile fibrosarcoma” “congenital fibrosarcoma”“婴儿型纤维肉瘤”,并从中筛选肠道婴儿型纤维肉瘤的病例。
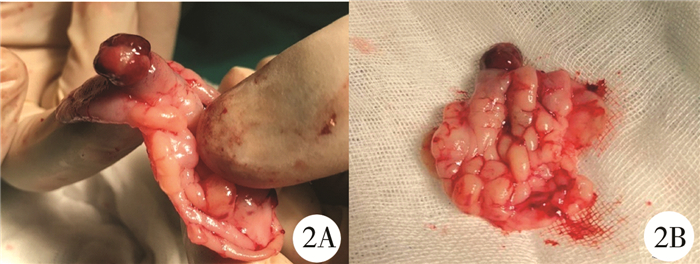
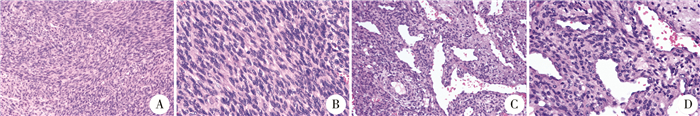
结果 一、病理检查肉眼见灰粉质软肠管一段,肠壁不规则增厚,增厚区旁可见息肉样隆起1枚,大小为1.0 cm×0.7 cm×0.6 cm(图 2)。镜下见肿瘤主要位于肠壁黏膜下,局部浸润肌层至浆膜外,呈实性、弥漫分布,瘤细胞呈梭形或卵圆形,局部排列呈束状或鲱鱼骨状,核浆比高,病理性核分裂像易见,间质中见扩张的不规则分支血管(图 3)。免疫组化:Vimentin(+),SMA(局灶+),Catenin(+),CD99(+),Desmin(-),CD117(-),DOG1(-),CD34(-),ALK(-),INI-1(+),CK(AE1/AE3)(-),EMA(-),S-100(-),Ki-67(15%+),CD21(-),TLE1(+),Calponin(-),WT1(-),EBER(-),BcL-2(-),STAT6(-),SOX10(-)。荧光原位杂交(fluorescence in situ hybridization, FISH):染色体ETV6-NTRK3基因位点无断裂易位。
|
图 2 婴儿型纤维肉瘤患者切除肿瘤及病变肠管 Fig.2 Lesion resection and gross inspection |
|
图 3 婴儿型纤维肉瘤患者病理形态学结果 Fig.3 Histopathological examination 注 A:致密的梭形细胞呈束状交错排列,呈“鲱鱼骨样”结构(HE染色,×200);B:核浆比高,易见病理性核分裂像(HE染色,×200);C和D:间质中见扩张的不规则分支血管(HE染色,×400) |
患者未予放疗或化疗。术后1个月、3个月、6个月门诊复查,未出现便血等不适,复查血常规无贫血,复查腹部超声未见复发与转移,需进一步长期随访。
三、文献分析文献检索发现截止2019年9月,仅有19例肠道婴儿型纤维肉瘤病例报道,均为国外文献,结合本例共20例,临床资料见表 1。其中12例肿瘤(12/20, 60.0%)位于小肠(回肠8例,空肠3例,十二指肠1例),6例(6/20, 30.0%)位于结肠(乙状结肠3例,结肠脾曲2例,结肠肝曲1例),2例(2/20, 10.0%)位于回盲部。男童11例(55.0%),女童9例(45.0%)。患者均行手术治疗,其中14例行开腹肠切除肠吻合术,3例行腹腔镜探查+肠切除肠吻合术,2例行开腹肿瘤切除+肠造瘘术,1例行开腹手术切除+二次手术+辅助化疗。患者手术切除肿瘤的平均长径为35.4 mm,有2例长径不详。除1例远端肠系膜淋巴结转移及1例未提及外,随访3~300个月后,所有患者预后良好,无转移复发。
| 表 1 截至2019年9月文献报道的肠道IFS临床资料 Table 1 Clinical profiles of intestinal IFS reported in literature up until September 2019 |
|
|
婴儿型纤维肉瘤(infantile fibrosarcoma, IFS)是一种罕见的软组织肉瘤,最早由Stout于1962年报道,发病率约5/1 000 000[12, 17]。世界卫生组织界定5岁以下的纤维肉瘤为IFS,约80%的患者在1岁内诊断,约40%的患者于出生时诊断[18]。回顾肠道IFS,通常于新生儿早期发病,出生后7 d内发病者占80%(16/20),其中5例在出生时即发病。目前IFS的病因尚不明确,有报道称与母亲孕期接触石油制品有关[20]。
婴儿型纤维肉瘤通常生长于皮下组织,表现为无痛性肿块,在四肢远端最为常见,其次是躯干中轴部位和头颈部,也有报道发病于腹膜后、肠系膜、口腔等部位[18, 19]。发病于肠道的婴儿型纤维肉瘤非常少见,回顾本研究报道的肠道IFS,多位于小肠,其次为结肠、回盲部,临床表现以肠穿孔、肠梗阻为主,穿孔可能是肿瘤破裂和肠壁缺血所致,可引发胎粪性腹膜炎。瘤体巨大者,患者以腹胀为首发表现,多为不全梗阻。位于回盲部的IFS需格外关注,Obayashi等[8]报道一例回盲部IFS引发肠套叠。Zeytun等[7]报道一例体格检查发现的无症状回盲部IFS,肿瘤直径55 mm,远大于表现穿孔和梗阻的IFS(平均直径分别为17.4 mm和20.7 mm),其阴性症状可能与回盲部的特殊位置有关。此外,Kim等[6]报道一例结肠IFS,仅表现为气腹,考虑可能存在肠壁的微小穿孔。本例患者肿瘤长径10 mm,是首例以单纯便血为表现的肠道IFS,可能与肿瘤上皮受损有关。婴幼儿期便血的常见原因包括坏死性小肠结肠炎、肠扭转、牛奶过敏、梅克尔憩室炎、肠重复畸形等,以肿瘤发病的病例极少,因此对于不明原因的婴幼儿便血,需警惕肠道IFS[21]。
常用的影像学检查包括超声、CT和MRI,但其特异性差,诊断价值有限。IFS的超声表现多为低回声或无回声的软组织包块,周围血流信号增加[22]。产检超声有一定的提示作用,Parmar等[5]报道一例长径130 mm的结肠IFS,产检超声发现羊水过多;Shima等[14]报道一例肠穿孔致胎粪性腹膜炎的空肠IFS,产检超声发现羊水过多和胎儿腹水。关于CT的影像学特点描述较少,本例患者行增强CT后发现肠腔肿物,不规则强化,由肠系膜下动脉分支供血。MRI对软组织的成像较好,是首选的影像学检查,Lee等[22]报道四肢IFS表现为T1低信号和T2高信号的软组织包块,周围可见血管信号,注射造影剂后呈不均匀强化。
病理检查是诊断婴儿型纤维肉瘤的金标准,需结合组织学、免疫组化和分子诊断。显微镜下可见致密的梭形细胞呈束状排列,形成“鲱鱼骨样”结构,有丝分裂程度高,周围血管丰富,可伴有出血、坏死等,与本例的组织学特征一致。IFS需与其他梭形细胞肿瘤如炎性肌纤维母细胞瘤、婴幼儿纤维瘤病、梭形细胞横纹肌肉瘤、胃肠道间质瘤等相鉴别。免疫组化结果中,IFS通常表现为Vimentin(+),部分病例表现为SMA(+)、H-caldesmon(+),其余Desmin、Myogenin、S-100、CD117、CD34等多为阴性,与本例患者相符[13]。分子诊断是IFS的重要诊断工具,IFS存在染色体易位(12; 15)(p13; q25),可将转录调节因子(ETV6)的二聚结构域与膜受体酪氨酸激酶(NTRK3)融合[24]。最近有报道在炎性肌纤维母细胞瘤中也发现ETV6-NTRK3异位,但其ALK呈阴性,可综合判断[25]。本例患者未发现ETV6-NTRK3异位,而回顾所有病例发现,ETV6-NTRK3异位阳性率为46.1%(6/13)。Berrebi等[13]指出存在一种发病于肠道的临床病理类型,其组织学与IFS相似,但没有ETV6-NTRK3异位。本例患者结合组织学、免疫组化和分子诊断,经3家医院病理科联合会诊后,诊断为婴儿型纤维肉瘤。
婴儿型纤维肉瘤的主要治疗方法为根治性手术切除,如果切缘病理为阴性,则无需化疗或放疗[20, 26]。当影响重要器官或肿瘤因体积过大而不能完整切除时,可行辅助化疗,常用的化疗方案包括VAC(长春新碱、放线菌素D、环磷酰胺)、AVCP方案(阿霉素、长春新碱、环磷酰胺、顺铂)等。回顾肠道IFS病例, 大部分患者(14/20, 70.0%)行开腹探查肠切除肠吻合术,其中1例因感染严重行肠切除后造瘘术,1例因肿瘤过大止血困难,行肠造瘘术并分期切除。1例首次手术肿物切缘阳性,残留灶继发肠梗阻而行二次手术,病理活检提示肠系膜远端淋巴结转移,予长春新碱、阿霉素和环磷酰胺化疗。2例有肠梗阻表现的患者行腹腔镜探查+肠切除肠吻合术。最佳手术方式的选择需结合患者病情及腹部情况综合考虑,如患者病情危重、瘤体过大、腹腔粘连严重,首选开腹手术,必要时行分期手术或肠造瘘术。如不能判定肿物边界,术中务必行冰冻病理检查以完整切除。本例患者术前影像学检查提示肿物位于右下腹,因其性质未定而行腹腔镜手术,反复探查后确定病变位于左下腹乙状结肠和降结肠交界处,未见其他病灶,扩大切口将其提出并完整切除。术前与术中病变位置不一,可能与乙状结肠活动致位置改变有关,如盲目开腹手术会增加手术难度和对患者的创伤。因此,建议病情平稳且腹部粘连少的患者可先行腹腔镜探查。
与成人纤维肉瘤相比,婴儿型纤维肉恶性程度低,预后更好,5年生存率约为90%,转移以躯干IFS的轴向转移为主,转移率为5%,原位复发率为5% ~40%[27, 28]。20例肠道IF患者随访3~300个月后,除1例发生肠系膜远端淋巴结转移予辅助化疗外,其余患者未行化疗或放疗,未发现复发和转移。本例患者未予放疗或化疗,预后良好,将继续随访监测病情。
综上所述,肠道的婴儿型纤维肉瘤是一种罕见的软组织肉瘤。本文报道了国内首例肠道婴儿型纤维肉瘤并回顾相关文献,旨在加深对于本病认识,以减少误诊。肠道IFS多于新生儿早期发病,主要表现为肠穿孔和肠梗阻,可结合患者病情选择合适的手术方案,必要时行辅助化疗。肠道IFS恶性程度低,完整切除后预后良好,复发和转移少。IFS常伴有ETV6-NTRK3异位,但在肠道IFS中ETV6-NTRK3异位比例不高,因此需结合组织学、免疫组化、分子诊断等进一步明确诊断。
| [1] |
Al-Salem AH. Congenital-infantile fibrosarcoma masquerading as sacrococcygeal teratoma[J]. J Pediatr Surg, 2011, 46(11): 2177-2180. DOI:10.1016/j.jpedsurg.2011.08.011 |
| [2] |
van Niekerk ML, Nel WA, Slavik T. Infantile fibrosarcoma of the ileum presenting with congenital bowel obstruction[J]. J Pediatr Surg, 2010, 45(2): 461-462. DOI:10.1016/j.jpedsurg.2009.10.092 |
| [3] |
Islam S, Soldes OS, Ruiz R, et al. Primary colonic congenital infantile fibrosarcoma presenting as meconium peritonitis[J]. Pediatr Surg Int, 2008, 24(5): 621-623. DOI:10.1007/s00383-008-2113-0 |
| [4] |
Buccoliero AM, Castiglione F, Rossi Degl'Innocenti D, et al. Congenital/Infantile fibrosarcoma of the colon:morphologic, immunohistochemical, molecular, and ultrastructural features of a relatively rare tumor in an extraordinary localization[J]. J Pediatr Hematol Oncol, 2008, 30(10): 723-727. DOI:10.1097/MPH.0b013e31817541df |
| [5] |
Parmar V, Peters RT, Cheesman E, et al. Congenital infantile fibrosarcoma of the colon:a case series and literature review[J]. Pediatr Surg Int, 2014, 30(10): 1079-1085. DOI:10.1007/s00383-014-3589-4 |
| [6] |
Kim HY, Cho YH, Byun SY, et al. A case of congenital infantile fibrosarcoma of sigmoid colon manifesting as pneumoperitoneum in a newborn[J]. J Korean Med Sci, 2013, 28(1): 160-163. DOI:10.3346/jkms.2013.28.1.160 |
| [7] |
Zeytun H, Okur MH, Basuguy E, et al. Congenital-infantile fibrosarcoma of the ileocecal region:the first case presentation[J]. Pediatr Surg Int, 2016, 32(1): 97-99. DOI:10.1007/s00383-015-3802-0 |
| [8] |
Obayashi J, Koizumi H, Hoshikawa M, et al. A case of congenital infantile fibrosarcoma of the bowel presenting as a neonatal intussusception[J]. Pathol Int, 2017, 67(12): 644-648. DOI:10.1111/pin.12603 |
| [9] |
Scirè G, Mantovani A, Zampieri N, et al. Transumbilical laparoscopic treatment of Congenital Infantile Fibrosarcoma of the Ileum[J]. Pediatr Med Chir, 2014, 36(4): 93. DOI:10.4081/pmc.2014.93 |
| [10] |
Rizkalla H, Wildgrove H, Quinn F, et al. Congenital fibrosarcoma of the ileum:case report with molecular confirmation and literature review[J]. Fetal Pediatr Pathol, 2011, 30(3): 156-160. DOI:10.3109/15513815.2010.547554 |
| [11] |
Bruno C, Caliari G, Zampieri N, et al. Congenital fibrosarcoma of the bowel:sonographic description of a rare case of neonatal intestinal obstruction[J]. J Clin Ultrasound, 2014, 42(6): 363-366. DOI:10.1002/jcu.22117 |
| [12] |
See WSQ, Cheuk DKL, To KF, et al. Congenital intestinal fibrosarcoma with rapid recurrence requiring adjuvant chemotherapy[J]. Pediatr Int, 2017, 59(6): 733-736. DOI:10.1111/ped.13252 |
| [13] |
Berrebi D, Fournet JC, Boman F, et al. Intestinal congenital/infantile fibrosarcoma:a new clinico-pathological entity?[J]. Pediatr Surg Int, 2015, 31(4): 375-379. DOI:10.1007/s00383-015-3670-7 |
| [14] |
Shima Y, Ikegami E, Takechi N, et al. Congenital fibrosarcoma of the jejunum in a premature infant with meconium peritonitis[J]. Eur J Pediatr Surg, 2003, 13(2): 134-136. DOI:10.1055/s-2003-39560 |
| [15] |
Kaiser M, Liegl-Atzwanger B, Nagy E, et al. Congenital Infantile Fibrosarcoma Causing Intestinal Perforation in a Newborn[J]. Case Rep Pediatr, 2017, 2017: 2969473. DOI:10.1155/2017/2969473 |
| [16] |
Shearburn EW, Teja K, Botero LM, et al. Pancreaticoduodenectomy in the treatment of congenital fibrosarcoma of the duodenum[J]. J Pediatr Surg, 1975, 10(5): 801-806. DOI:10.1016/0022-3468(75)90388-7 |
| [17] |
Stout AP. Fibrosarcoma in infants and children[J]. Cancer, 1962, 15(5): 1028-1040. DOI:10.1002/1097-0142(196209/10)15:53.0.CO;2-X |
| [18] |
Steelman C, Katzenstein H, Parham D, et al. Unusual presentation of congenital infantile fibrosarcoma in seven infants with molecular-genetic analysis[J]. Fetal Pediatr Pathol, 2011, 30(5): 329-337. DOI:10.3109/15513815.2011.587497 |
| [19] |
Orbach D, Rey A, Cecchetto G, et al. Infantile fibrosarcoma:management based on the European experience[J]. J Clin Oncol, 2010, 28(2): 318-323. DOI:10.1200/jco.2009.21.9972 |
| [20] |
Ortega-Garcia JA, Soldin OP, Lopez-Hernandez FA, et al. Congenital fibrosarcoma and history of prenatal exposure to petroleum derivatives[J]. Pediatrics, 2012, 130(4): e1019-e1025. DOI:10.1542/peds.2011-1307 |
| [21] |
Romano C, Oliva S, Martellossi S, et al. Pediatric gastrointestinal bleeding:Perspectives from the Italian Society of Pediatric Gastroenterology[J]. World J Gastroenterol, 2017, 23(8): 1328-1337. DOI:10.3748/wjg.v23.i8.1328 |
| [22] |
Braun P, Fernández-Montes JG, Calatayud AV. Congenital infantile fibrosarcoma:Report of four cases and review of the literature[J]. Eur J Radiol Extra, 2007, 61(1): 33-39. DOI:10.1016/j.ejrex.2006.10.004 |
| [23] |
Lee MJ, Cairns RA, Munk PL, et al. Congenital-infantile fibrosarcoma:magnetic resonance imaging findings[J]. Can Assoc Radiol J, 1996, 47(2): 121-125. |
| [24] |
Knezevich SR, McFadden DE, Tao W, et al. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma[J]. Nat Genet, 1998, 18(2): 184-187. DOI:10.1038/ng0298-184 |
| [25] |
Alassiri AH, Ali RH, Shen Y, et al. ETV6-NTRK3 Is Expressed in a Subset of ALK-Negative Inflammatory Myofibroblastic Tumors[J]. Am J Surg Pathol, 2016, 40(8): 1051-1061. DOI:10.1097/pas.0000000000000677 |
| [26] |
Hayes-Jordan A. Recent advances in non-rhabdomyosarcoma soft-tissue sarcomas[J]. Semin Pediatr Surg, 2012, 21(1): 61-67. DOI:10.1053/j.sempedsurg.2011.10.006 |
| [27] |
Thebaud E, Mezel A, Leroy X, et al. Fibrosarcoma in children and adolescents:different entities for the same name[J]. Bull Cancer, 2012, 99(6): 715-722. DOI:10.1684/bdc.2012.1597 |
| [28] |
Bourgeois JM, Knezevich SR, Mathers JA, et al. Molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors[J]. Am J Surg Pathol, 2000, 24(7): 937-946. DOI:10.1097/00000478-200007000-00005 |
 2020, Vol. 19
2020, Vol. 19


