Xp11.2易位TFE3基因融合相关性肾癌简称Xp11.2易位性肾癌,是肾癌的一种少见亚型,2004年首次于世界卫生组织肾癌病理组织学分类中出现,2016年被纳入家族性MiT易位性肾细胞癌[1]。Xp11.2易位性肾癌较罕见,主要发生于儿童和青少年,成人中女性多于男性,儿童患者中男女性差别不大,儿童肾癌中约1/3为Xp11.2易位性肾癌[2, 3]。本研究回顾性分析湖南省儿童医院2011年1月至2019年6月经手术病理确诊的3例儿童Xp11.2易位性肾癌CT表现,并结合相关文献进行分析,探讨CT对该病的诊断价值及鉴别诊断,以提高诊断的准确性。
材料与方法 一、一般资料收集2011年1月至2019年6月湖南省儿童医院经手术病理确诊的3例Xp11.2易位TFE3基因融合相关性肾癌患儿临床资料,其中男童2例,女童1例,年龄分别为2岁1个月、9岁8个月、2岁9个月。临床表现:2例出现无痛性血尿,1例腰部及腹部疼痛。3例均行根治性手术治疗,完整切除病灶后病理证实为Xp11.2易位TFE3基因融合相关性肾癌。
二、CT检查采用飞利浦64排Brilliance双螺旋CT扫描仪,扫描范围从膈顶至盆腔膀胱水平,行肾脏多期动态增强扫描。扫描参数:管电压120 kV,管电流80~100 mA,层厚层间距5 mm,螺距1.5。动态增强扫描时自肘静脉经高压注射器注射非离子碘对比剂碘海醇(300 mg I/mL),1.0~1.5 mL/kg,流速为0.5~2.0 mL/s。利用系统重建软件行冠矢状位图像重建。
三、图像分析由两名经验丰富的儿童腹部专业放射科医师共同阅片,当诊断意见不一致时,由两名医师协商决定。重点观察病灶的部位(右肾、左肾或双肾)、大小(最大横截面测量)、形态(类圆形或不规则形)、钙化(CT值>130 HU)、出血(CT值50~60 HU)、囊变坏死(CT值0~20 HU)、强化程度方式(皮髓质期强化较平扫CT值增加10~20 HU为轻度强化,增加21~40 HU为中度强化,>40 HU为明显强化)以及转移情况等。
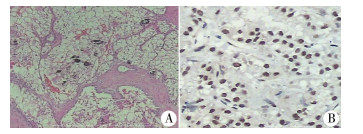
四、病理检查肉眼观察:肿瘤切面多呈灰白、灰褐色,质软,局部可见出血、坏死、囊变等。部分肿瘤与肾实质边界欠清晰。镜下:肿瘤细胞胞质丰富,大部分为胞浆透亮癌细胞,部分为嗜酸性颗粒状胞浆,肿瘤细胞排列呈实性巢状、乳突状和假乳突状,核仁大且明显,瘤巢区域见砂砾体,伴出血坏死。免疫组化:TFE3(2+)、Ki-67(5%+)、Vim(3+)、CK(区域+)、INI-1(3+)均阳性。1例EMA(灶+),1例CEA(灶+),1例CD10(3+),2例CyclinD1(2+)(图 1)。
|
图 1 Xp11.2易位TFE3基因融合相关性肾癌病理检查结果 Fig.1 Histopathological examination of renal cell carcinoma associated with Xp11.2 translocation/FE3 gene fusions 注 A:镜下肿瘤呈乳头状,瘤体细胞胞浆大部分为透亮的癌细胞,部分有嗜酸性颗粒状胞浆,核仁大、明显,区域见砂砾体,伴出血坏死(HE染色,×100); B:TFE3免疫组化染色,镜下瘤体细胞呈棕褐色阳性染色(×100) |
通过检索万方、CNKI、迈特思创、Pubmed数据库,检索关键词:Xp11.2易位TFE3基因融合相关性肾癌(renal cell carcinoma associated with Xp11.2 translocation/ TFE3 gene fusion)。剔除成人(>18岁)病例及无法获得临床、影像学资料的儿童病例,共检索出16例。
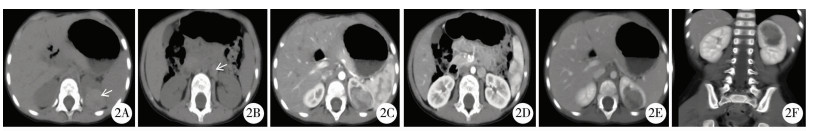
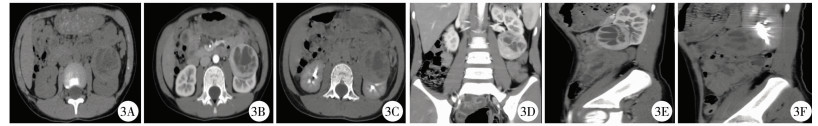
结果 一、本研究及文献病例CT表现19例均为单发病灶,其中右肾10例,左肾9例,19个病灶大小不等,最大横径为2.1~17.2 cm,平均5.7 cm。4例位于肾髓质内,14例位于肾皮髓质内,1例位于皮质。12例呈类圆形,7例呈不规则形,部分可见分叶。CT平扫8例呈稍高密度(8/15,53.3%),7例呈等低密度(7/15,46.7%); 12例出现钙化(12/19,63.2%); 10例合并出血(10/19,52.6%); 16例出现囊变坏死(16/19,84.2%)。4例MRI检查示T1WI及T2WI均呈不均匀信号,1例中度不均匀持续强化,1例明显不均匀持续强化。CT动态增强扫描示病灶强化不均匀,6例轻度强化,4例中度强化,6例明显强化。肿瘤实性部分各期强化程度均低于正常肾皮质,6例肿瘤实性部分皮髓质期及延迟期呈渐进式持续强化(6/16,37.5%),8例肿瘤实性部分皮质期强化高于髓质期,延迟期强化稍低于髓质期,强化程度呈逐渐减低趋势(8/16,50.0%); 2例各期强化无明显变化(2/16,12.5%)(图 2~图 4)。13例见完整或不完整“假包膜征” (13/19,68.4%)。10例出现腹膜后及腹主动脉旁淋巴结转移(10/19,52.6%),部分淋巴结可见钙化,1例肝脏受累,1例颈部淋巴结转移,4例腔静脉受累,1例2个月后子宫转移,1例死亡(表 1)。
|
图 2 Xp11.2易位TFE3基因融合相关性肾癌CT图 Fig.2 CT features of renal cell carcinoma associated with Xp11.2 translocation/FE3 gene fusions 注 病例1,因无痛性血尿6 d入院,B超发现左肾占位3 d。2A:CT平扫轴位示左肾肿块,病灶中心位于肾髓质内,平扫密度稍增高(箭头处,CT值约56 HU); 2B:肾门及腹膜后区可见肿大淋巴结影(箭头处); 2C、2D:动态增强扫描示肿块实性部分可见明显不均匀强化(CT值约121HU),强化程度低于肾皮质,高于肾髓质; 2E:延迟期示实性部分强化稍减低(CT值约108 HU),低于皮髓质,边界清晰,可见“假包膜征”; 2F:冠状位重建示病变侵犯左肾髓质 |
|
图 3 Xp11.2易位TFE3基因融合相关性肾癌CT图 Fig.3 CT features of renal cell carcinoma associated with Xp11.2 translocation/FE3 gene fusions 注 病例2,因腹痛、腰痛10余天入院。3A:CT平扫轴位示左肾肿块,呈囊实性密度,囊性CT值约14 HU,实性CT值约43 HU; 3B:动态增强扫描示肿块实性部分呈明显不均匀强化(CT值约140 HU),强化达高峰值,程度低于肾皮质,高于肾髓质; 3C:延迟期示肿块实性部分强化稍减低(CT值约71 HU),低于皮髓质,边界清晰,可见“假包膜征”; 3D、3E、3F:冠矢状位重建示病变外生型、主要侵犯左肾皮质 |

|
图 4 Xp11.2易位TFE3基因融合相关性肾癌CT图 Fig.4 CT features of renal cell carcinoma associated with Xp11.2 translocation/FE3 gene fusions 注 病例3,因无痛性血尿4 d入院,B超发现左肾占位3 d。4A:CT平扫轴位示左肾肿块,病灶中心位于肾髓质内,平扫密度稍增高(白箭头,CT值约51 HU),腹膜后可见肿大淋巴结并钙化(黑箭头); 4B:动态增强扫描示肿块实性部分呈明显不均匀强化(CT值约118 HU),实性部分强化达高峰值,程度低于肾皮质; 4C:延迟期示肿块实性部分强化程度较前稍均匀、减低(CT值约65 HU),低于皮髓质,边界清晰,可见“假包膜”征; 4D、4E、4F:冠状位重建示病变侵犯左肾髓质,边缘见小点片状钙化灶(白箭头) |
| 表 1 截至2019年11月文献报道的儿童Xp11.2易位性肾癌影像学表现 Table 1 Computed tomography manifestations of pediatric Xp11.2 translocation RCC reported in literature up until November 2019 |
|
|
临床上Xp11.2易位性肾癌非常罕见,本病主要发生于儿童和年轻女性,而其他常见类型肾癌主要发生于成年人[14]。有研究者发现该病的发生可能与儿童时期化疗史有关,但本研究病例均无化疗史,检索文献中有1例因淋巴瘤化疗时发现右肾占位[4, 15]。该病临床表现无特异性,可表现为肉眼无痛性血尿、腰痛、腹部肿块等,部分为偶然发现,本组病例中有4例患儿出现无痛性血尿,8例表现为腰痛及腹痛,4例触及腹部包块,1例尿失禁,1例因病毒性脑炎住院、1例因肺炎住院,与文献报道相符[16]。
二、CT表现及特征① 形态及部位:儿童Xp11.2易位TFE3基因融合相关性肾癌多起源于肾髓质,多为类圆形或不规则形,瘤体大时突破包膜向外生长,边界尚清晰,即“假包膜征”[17]。瘤体较大时可压迫邻近血管。4例病变中心位于髓质内,14例位于皮髓质,1例位于皮质。②密度:Xp11.2易位性肾癌平扫时多呈稍高密度影,此征象可作为诊断Xp11.2易位性肾癌的重要诊断依据之一。这可能是因为肿瘤内含丰富蛋白或伴有出血、含铁血黄素沉积。本组病例中有8例平扫密度稍高(8/15, 53.3%),与文献报道相符[18]。7例呈等低密度影。③钙化:Koo等[4]报道肿瘤中心及周边点状、斑片状钙化灶可提示Xp11.2易位性肾癌,钙化形态可表现为沙粒状、弧形蛋壳样及星芒样等多种形式,其钙化率高于其它亚型肾癌[19]。另有文献报道当儿童或青少年患肾脏肿瘤,平扫瘤体呈稍高密度影且出现钙化灶时,应高度怀疑Xp11.2易位性肾癌。本文中共有12例出现不同形态钙化灶(12/19,63.2%),此征象与文献报道一致[5]。④囊变与坏死:瘤体体积较大时易出现囊变、坏死,瘤体较小时囊变、坏死少见。本组囊变坏死较多见,其中有16例出现囊变坏死(16/19,84.2%),笔者分析可能是由于Xp11.2易位性肾癌轻中度血供较多见,瘤内更易出现坏死,此征象与国内外文献报道相符[20, 21]。⑤强化方式:增强扫描示实性部分均呈不均匀强化,实性部分各期强化程度均较正常肾皮质低,其中轻中度强化10例,明显强化6例。本文中动态增强扫描肿瘤强化方式主要有三类:第一,6例肿瘤实性部分皮髓质期及延迟期呈渐进式持续强化(6/16,37.5%)[15, 18]; 与卢伟光等[22]报道的“快进慢出”、渐进式强化方式相符。第二,8例肿瘤实性部分皮质期强化高于髓质期,延迟期强化稍低于髓质期,强化程度呈逐渐减低趋势(8/16,50.0%),与张旭婷等[23]、Chen等[24]报道的强化方式类似,本院3例肿瘤均属于此类型强化方式; 第三,2例动态增强各期强化无明显变化(2/16,12.5%)[10, 13]。有文献报道囊实性肿块不均匀强化,呈结节状、分隔样强化[25]。笔者分析儿童Xp11.2易位性肾癌强化方式多样,但以轻中度强化为主,主要原因可能与肿瘤内轻中度供血模式、肿瘤细胞的成分及排列有关,此外可能还与CT检查仪器及参数有关。延迟期肿瘤边界清晰、呈“假包膜征”改变,此征象可作为诊断Xp11.2易位性肾癌的又一重要诊断依据。本文中有13例出现该征象(13/19,68.4%),与文献报道一致[26]。⑥MRI表现:检索文献中有4例肿瘤MRI信号不均匀,T1WI呈不均匀稍高信号,T2WI呈不均匀稍低信号,提示瘤体内出血,可能与含铁血黄素沉积有关,增强呈不均匀渐进式持续性强化改变[6-8]。⑦转移情况:Xp11.2易位性肾癌易出现转移,以淋巴结转移多见,预后较差,当儿童或年轻女性肾脏肿瘤出现转移时可提示该病[6, 8]。本组病例中10例出现腹膜后腹主动脉旁淋巴结转移,并部分淋巴结钙化,1例肝脏受累,1例出现颈部淋巴结转移,4例出现腔静脉癌栓,当有癌栓形成时则提示预后较差,1例随访2个月后出现子宫转移,1例死亡[27, 28]。
三、鉴别诊断儿童Xp11.2易位性肾癌主要需要与以下肿瘤鉴别:①肾母细胞瘤:其是儿童肾脏最常见的肿瘤。影像学上儿童Xp11.2易位性肾癌与肾母细胞瘤难以鉴别,最终依靠病理检查来鉴别。前者发病年龄一般较后者大,前者常因血尿发现,后者常因触及包块而发现。平扫时前者密度稍高,且钙化更常见,动态增强各期强化程度均低于肾皮质,高于肾髓质,呈渐进式持续强化或延迟期强化程度稍减低,强化程度一般高于肾母细胞瘤。②乳头状肾细胞癌:儿童中极少见,为乏血供肿瘤,增强各期强化程度均低于正常肾皮质,强化程度低于Xp11.2易位性肾癌[29]。③肾透明细胞肉瘤:比较罕见,2岁为发病高峰期,易发生骨转移。液化坏死范围更大、更常见,血供丰富,实性部分强化明显,呈云絮状、虎斑样改变[30]。④肾横纹肌样瘤:为高度恶性肿瘤,较早发生转移。肿瘤主要位于肾中心且易累及肾门,呈膨胀性生长,包膜下积液,呈分叶状改变,常伴有颅内肿瘤。
综上,儿童Xp11.2易位性肾癌临床上罕见,若儿童肾脏肿瘤主要位于皮髓质内,平扫密度稍高,钙化多见且形态多样,有出血性坏死囊变,增强呈轻中度不均匀强化,且各期强化程度均低于肾皮质,高于肾髓质,呈渐进式持续性强化或延迟期强化程度稍减低趋势,出现淋巴结转移,应考虑Xp11.2易位TFE3基因融合相关性肾癌的可能。
| [1] |
Humphrey PA, Moch H, Cubilla AL, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Prat B:Prostate and Bladder Tumors[J]. Eur Urol, 2016, 70(1): 106-119. DOI:10.1016/j.eururo.2016.02.028 |
| [2] |
Cheng XM, Gan WD, Zhang GT, et al. Clinicai characteristics of Xp11.2 translocation/TFE3 gene fusion renal cell carcinoma:a systematic review and meta-analysis of observational studies[J]. BMC Urol, 2016, 16(1): 40. DOI:10.1186/s12894-016-0154-6 |
| [3] |
Winarti NW, Argani P, De Marzo AM, et al. Pediatric renal cell carcinoma associated with Xp11.2 translocation/TEF3 gene fusion[J]. Int J Surg Pathol, 2008, 16(1): 66-72. DOI:10.1177/1066896907304994 |
| [4] |
Koo HJ, Choi HJ, Kim MH, et al. Radiologic-pathologic correlation of renal cell carcinoma associated with Xp11.2 translocation[J]. Acta Radiol, 2013, 54(7): 827-834. DOI:10.1177/0284185113484019 |
| [5] |
He J, Huan Y, Qiao Q, et al. Renal carcinomas associated with Xp11.2 translocation:are CT findings suggestive of the diagnosis?[J]. J Clin Radiol, 2014, 69(1): 45-51. DOI:10.1016/j.crad.2013.08.004 |
| [6] |
Liu KF, Xie P, Peng WJ, et al. Renal carcinomas associated with Xp11.2 translocations/TFE3 gene fusions:Findings on MRI and computed tomography imaging[J]. J Magnetic Resonance Imaging, 2014, 40(2): 440-447. DOI:10.1002/jmri.24349 |
| [7] |
Kato H, Kanematsu M, Yokoi S, et al. Renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion:Radiological findings mimicking papillary subtype[J]. J Magnetic Resonance Imaging, 2011, 33(1): 217-220. DOI:10.1002/jmri.22392 |
| [8] |
Wang W, Ding JH, Li Y, et al. Magnetic resonance imaging and computed tomography characteristics of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion[J]. PLoS ONE, 2014, 9(6): e99990. DOI:10.1371/journal.pone.0099990 |
| [9] |
张雪, 周胜利, 苗重昌. XP11.2易位/TFE3基因融合相关性肾癌的CT诊断及鉴别诊断[J]. 医学影像学杂志, 2015, 25(6): 1088-1090. Zhang X, Zhou SL, Miao CC. The CT features and the differential diagnosis of the renal cell carcinoma associated with XP11.2 translocation/TFE3 gene fusions[J]. J Med Imaging, 2015, 25(6): 1088-1090. (in Chinese) |
| [10] |
魏维, 夏天. Xp11.2易位/TFE3基因融合相关性肾癌1例及文献分析[J]. 国际病理科学与临床杂志, 2013, 33(3): 268-272. Wei W, Xia T. Renal carcinoma associated with Xp11.2 translocation/TFE3 gene fusion:a case report and literature review[J]. Int J Pathol Clin Med, 2013, 33(3): 268-272. DOI:10.3969/j.issn.1673-2588.2013.03.017 (in Chinese) |
| [11] |
尼玛, 钟定荣, 次旦旺久, 等. Xp11.2易位/TFE3基因融合相关性肾癌2例CT表现及病理对照分析并文献复习[J]. 实用放射学杂志, 2018, 34(1): 163-165. Ni M, Zhong DR, Ci DWJ, et al. CT features and pathological analysis of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions:two case reports and literature review[J]. J Pract Radiol, 2018, 34(1): 163-165. DOI:10.3969/j.issn.1002-1671.2018.01.051 (in Chinese) |
| [12] |
杜昕, 萨日, 关锋, 等. Xp11.2易位/TFE3基因融合相关性肾癌PET/CT显像及CT增强2例报道[J]. 重庆医学, 2018, 47(22): 124-126. Du X, Sa R, Guan F, et al. PET/CT imaging and enhanced CT of renal carcinoma associated with Xp11.2 translocations/TEF3 gene fusions:a report of two cases[J]. J Med Chongqing, 2018, 47(22): 124-126. DOI:10.3969/j.issn.1671-8348.2018.22.036 (in Chinese) |
| [13] |
于洋, 邢东亮, 康郑军, 等. 儿童和青少年Xp11.2易位/TFE3基因融合相关性肾癌2例报道并文献分析[J]. 肿瘤基础与临床, 2018, 31(3): 242-244. Yu Y, Xing DL, Kang ZJ, et al. Renal carcinoma associated with Xp11.2 translocations/TEF3 gene fusions in children and adolescents[J]. Jour Basic Clini Oncol, 2018, 31(3): 242-244. DOI:10.3969/j.issn.1673-5412.2018.03.019 (in Chinese) |
| [14] |
Kuroda N, Mikami S, Pan CC, et al. Review of renal carcinoma associated with Xp11.2 translocations/TEF3 gene fusions with focus on pathobiological aspect[J]. Histol Histopathol, 2012, 27(2): 133-140. DOI:10.14670/HH-27.133 |
| [15] |
Argani P, Lae M, Ballard ET, et al. Translocation carcinomas of the kidney after chemotherapy in childhood[J]. J Clin Oncol, 2006, 24(10): 1529-1534. DOI:10.1016/j.urolonc.2006.08.004 |
| [16] |
井颖, 顾涛, 王建林, 等. 小儿Xp11.2易位/TFE3基因融合相关性肾癌1例[J]. 临床小儿外科杂志, 2015, 14(2): 157-160. Jing Y, Gu T, Wang JL, et al. A case of renal cancer associated with Xp11.2 translocation/TFE3 gene fusion in children[J]. J Clin Ped Sur, 2015, 14(2): 157-160. DOI:10.3969/j.issn.1671-6353.2015.02.024 (in Chinese) |
| [17] |
朱庆强, 王中秋, 吴晶涛, 等. Xp11.2易位TFE基因融合相关性肾癌的多层螺旋CT表现[J]. 中华放射学杂志, 2012, 46(6): 516-520. Zhu QQ, Wang ZQ, Wu JT, et al. Multi-slice spiral CT findings of renal cell carcinoma associated with Xp11.2 translocation-TFE gene fusion[J]. J Chin Radiol, 2012, 46(6): 516-520. DOI:10.3760/cma.j.issn.1005-1201.2012.06.008 (in Chinese) |
| [18] |
高凯波, 赵秀丽, 叶慧义, 等. Xp11.2易位/TFE3基因融合相关性肾癌的CT与MRI表现[J]. 中国医学影像学杂志, 2017, 25(3): 222-226. Gao KB, Zhao XL, Ye HY, et al. CT and MRI findings of Renal Cell Carcinoma Associated with Xp11.2 Translocation/TFE3 Gene Fusions[J]. J Chin Med Imaging, 2017, 25(3): 222-226. DOI:10.3969/j.issn.1005-5185.2017.03.015 (in Chinese) |
| [19] |
He J, Zhou KF, Zhu B, et al. Dynamic Contrast-Enhanced CT Characterization of Xp11.2 Translocation/TFE3 Gene Fusions versus Papillary Renal Cell Carcinomas[J]. Bio Med Research Interna, 2015, 2015: 298679. DOI:10.1155/2015/298679 |
| [20] |
Dai CC, Sheng RF, Ding YQ, et al. Magnetic resonance imaging findings of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion in adults:a pilot study[J]. Abdomi Radiol(NY), 2019, 44(1): 209-217. DOI:10.1007/s00261-018-1703-0 |
| [21] |
陈伟, 史玉振, 王亚婷, 等. XP11.2易位/TFE3基因融合相关性肾癌的MSCT表现及病理特征性分析[J]. 临床放射学杂志, 2019, 38(7): 1278-1281. Chen W, Shi YZ, Wang YT, et al. The MSCT Features and Pathological Characteristics Analysis of the Renal Cell Carcinoma Associated with XP11.2 Translocation/TFE3 Gene Fusion[J]. J Clin Radiol, 2019, 38(7): 1278-1281. DOI:10.1343/j.cnki.jcr.20190731.034 (in Chinese) |
| [22] |
卢伟光, 彭洋, 孙炎平, 等. Xp11.2易位/TFE3基因融合相关性肾癌的CT和MRI表现[J]. 影像诊断与介入放射学, 2018, 27(3): 199-203. Lu WG, Peng Y, Sun YP, et al. CT and MRI findings of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion[J]. Diagn Imaging Interven Radiol, 2018, 27(3): 199-203. DOI:10.3969/j.issn.1005-8001.2018.03.005 (in Chinese) |
| [23] |
张旭婷, 任基伟, 靳宏星, 等. Xp11.2易位/TFE3基因融合相关性肾癌的影像诊断与鉴别诊断[J]. 医学影像学杂志, 2019, 29(6): 997-1001. Zhang XT, Ren JW, Jin HX, et al. CT and MRI imaging in diagnosing renal carcinoma associated with Xp11.2 translocation/TFE3 gene fusion[J]. J Med Imaging, 2019, 29(6): 997-1001. (in Chinese) |
| [24] |
Chen X, Zhu Q, Li B, et al. Renal cell carcinoma assiociated with Xp11.2 translocation/TFE gene fusion:imaging findings in 21 patients[J]. Euro Radiol, 2017, 27(2): 543-552. DOI:10.1007/s00330-016-4421-4 |
| [25] |
程瑾, 陈皓, 史景丽, 等. Xp11.2易位/TFE3基因融合相关性肾癌的CT和MRI表现[J]. 放射学实践, 2018, 33(8): 811-815. Cheng J, Chen H, Shi JL, et al. MRI and CT features of renal cell carcinoma associated with Xp11.2 translocation/FE3 gene fusions[J]. Radiol Pract, 2018, 33(8): 811-815. DOI:10.13609/j.cnki.1000-0313.2018.08.009 (in Chinese) |
| [26] |
Cheng XM, He J, Gan WD, et al. Psendocapsule of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion:a clue for tumor enucleation[J]. Int J Clin Exp Pathol, 2015, 8(5): 5403-5410. |
| [27] |
Downey RT, Dillman JR, Ladino-Torres MF, et al. CT and MRI appearances and radiologic staging of pediatric renal cell carcinoma[J]. Pediatr Radiol, 2012, 42(4): 410-417. DOI:10.1007/s00247-011-2319-5 |
| [28] |
Liu N, Wang Z, Gan WD, et al. Renal Cell Carcinoma Associated with Xp11.2 Translocation/TFE3 Gene Fusion:Clinical Features, Treatments and Prognosis[J]. PLoS ONE, 2016, 11(11): e0166897. DOI:10.1371/journal.pone.0166897 |
| [29] |
Zhu QQ, Zhu WR, Wu JT, et al. Differential diagnosis between renal cell carcinoma associated with XP11.2 translocation/TFE gene fusion and papillary renal cell carcinoma based on CT and MRI findings[J]. J Chin Radiol, 2014, 94(19): 1470-1472. DOI:10.3760/cma.j.issn.0376-2491.2014.19.010 |
| [30] |
肖伟强, 刘鸿圣, 黄莉, 等. 儿童肾透明细胞肉瘤CT及临床病理特点分析[J]. 中国临床医学影像杂志, 2018, 29(8): 599-601. Xiao WQ, Liu HS, Huang L, et al. Analysis of CT features and clinicopathological features of renal clear cell sarcoma in children[J]. J Chin Clin Med Imaging, 2018, 29(8): 599-601. DOI:10.12117/jccmi.2018.020 (in Chinese) |
 2020, Vol. 19
2020, Vol. 19


