CDC5L属于Prp19复合体成员,生理作用下与Prp19一起参与pre-mRNA的剪接,DNA的损伤修复和底物泛素化修饰。病理状态下则参与肿瘤的侵袭,细胞周期的调控,抑制UV诱导的细胞凋亡[1-5];CDC5L通过和细胞周期检查点蛋白相互作用,调控细胞分裂周期G2/M期的转变,从而影响细胞有丝分裂进程[6, 7]。CDC5L在多种肿瘤中呈现高表达,提示了它可能对肿瘤的发生发展产生影响,并进一步影响肿瘤的治疗效果[3, 8, 9]。本研究将神经母细胞瘤患儿按不同性别、年龄、是否有骨髓浸润、肿瘤临床分期(International Neuroblastoma Staging System,INSS)、肿瘤病理诊断、Shimada病理分类、危险度分级及脑脊液神经元烯醇化酶(neuron specific enolase,NSE)、乳酸脱氢酶(lactate dehydrogenase,LDH)是否正常进行分组,分析不同亚组中CDC5L的表达水平差异,初步揭示CDC5L与神经母细胞瘤患儿临床和预后之间的关系。
材料与方法 一、临床资料收集2012年9月至2015年2月在上海交通大学医学院新华医院小儿外科就诊,且病理诊断为神经母细胞瘤的62例患儿作为研究对象,其中男童35例,女童27例;诊断时年龄≥18个月43例,<18个月19例;原发肿瘤部位为颈部1例,后纵膈11例,腹膜后49例,盆腔1例;首发症状为发热、咳嗽13例,腹痛腹块、呕吐纳差33例,肢体疼痛、活动不利5例,呼吸困难1例,颈部肿块2例,眶周肿块、眼球外突2例,臀部肿块1例,其它5例;按照是否有骨髓浸润、临床分期(INSS分期)、肿瘤病理诊断、Shimada病理分类[良好组织学类型、组织结构良好型(favorable histology,FH)和不良组织学类型、组织结构不良型(unfavorable histology,UFH)]及初诊NSE正常与否进行分组。本研究中所有患儿初诊时LDH水平均高于正常值。2009年国际神经母细胞瘤危险组(The international neuroblastoma risk group, INRG)通过统计分析8 800例神经母细胞瘤患儿的LDH值发现,LDH≥587 U/L是不良预后的因素,故本研究将初诊时LDH水平的界值点定义为587 U/L[10]。同时,对所有患儿的肿瘤标本进行免疫组化染色(CDC5L),分析各组中CDC5L的表达情况。本研究中所有患儿均接受了规范化治疗,随访至2017年12月,随访信息完整的患儿共50例[11]。
二、标本处理及染色结果观察所有标本均取自手术时切除的肿瘤组织,经切片、10%多聚甲醛固定、石蜡包埋及免疫组化(CDC5L)染色后,在光学显微镜下观察肿瘤细胞着色数量。在高倍镜下随机选取5~10个视野,计数每1 000个肿瘤细胞中阳性染色细胞数,取平均值作为最终值,并计算阳性染色比例。根据着染细胞比例将CDC5L染色结果分为阴性(0% ~5%)、弱阳性(6% ~25%)、中度阳性(26% ~50%)及强阳性(>50%);将阴性、弱阳性、中度阳性和强阳性分别记为0级、1级、2级和3级。本研究将阴性和弱阳性定义为低表达组,中度阳性和强阳性定义为高表达组。
三、蛋白免疫印迹(Western Blot,WB)取患儿(7例NB和7例GNB)肿瘤组织标本进行裂解,将蛋白进行BCA法定量及变性,取等量的蛋白在10%的聚丙烯酰胺凝胶上进行电泳,将凝胶上蛋白电转到聚偏二氟乙烯(polyvinylidene fluoride,PVDF)膜上,再将PVDF膜在室温下于5%的牛血清白蛋白(bovine serum albumin,BSA)溶液中封闭1 h,弃去封闭液,加合适一抗溶液(CDC5L,Abcam(英国), 货号:ab129114, 1 : 1 000;GAPDH,CST(美国), 货号:5174,1 : 1 000),放置于4℃摇床过夜,回收一抗,TBST缓冲液(20 mM Tris, 150 mM NaCl, 0.05 % Tween-20,翊圣,中国)溶液洗膜3次,每次5 min,室温孵育二抗(山羊抗兔,翊圣(中国),货号:33101ES60,1 : 10 000稀释)1 h。再次用TBST洗膜3次,每次5 min,曝光,使用化学发光法观察蛋白条带。
四、统计学处理使用SPSS 20.0统计软件进行数据整理与分析。对等级资料采用Wilcoxon符号秩和检验。对于不符合正态分布的计量资料采用Mann-Whitney U检验。对不同性别、年龄、是否有骨髓浸润、肿瘤临床分期(INSS)、肿瘤病理诊断、Shimada病理分类、危险度分级及NSE、LDH是否正常进行分组,分析不同亚组中CDC5L高表达比例的差异采用χ2检验。采用单因素和多因素Cox回归模型来分析CDC5L表达水平、临床分期、危险度分级、Shimada病理分型对患儿生存的影响。采用Kaplan-Meier法计算CDC5L不同表达组的生存概率,并采用Log-rank检验进行生存曲线的差异性分析。以P<0.05为差异有统计学意义。
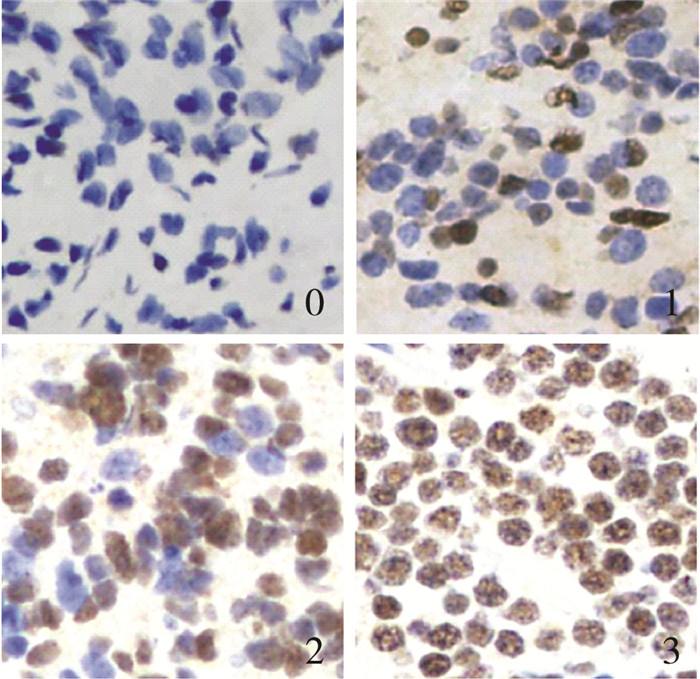
结 果 一、免疫组化发现CDC5L在神经母细胞瘤标本中存在差异表达使用神经母细胞瘤标本制成的组织芯片进行免疫组化染色,并初步分析CDC5L在神经母细胞瘤不同病理类型中的表达差异,可以看到CDC5L主要聚集在细胞核内,呈棕色染色,62例神经母细胞瘤标本中,根据前述分级标准,CDC5L高表达31例(50%),低表达31例(50%);CDC5L表达0级15例(24%),1级16例(26%),2级16例(26%), 3级15例(24%);依据病理检查结果诊断为NB患儿35例,CDC5L表达0级7例(20%),1级5例(14%),2级10例(29%),3级13例(37%);GNB患儿27例,CDC5L表达0级8例(30%),1级11例(41%),2级6例(22%),3级2例(7%)。对CDC5L染色分级结果进分析发现NB患儿CDC5L的表达较GNB患儿高(Z=-2.654,P=0.008),见图 1、表 1。
|
Download:
|
| 图 1 NB患儿肿瘤组织标本CDC5L不同表达水平 Fig. 1 Different expression levels of CDC5L in tissues of children with NB | |
|
|
表 1 CDC5L在NB患儿不同病理类型中差异性表达分析[n(%)] Table 1 Analysis of differential expression of CDC5L in diffe-rent pathological types of children with NB[n(%)] |
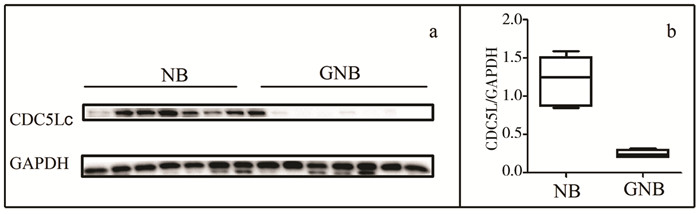
为了再次验证CDC5L在神经母细胞瘤不同病理类型中存在差异表达,取14例神经母细胞瘤患儿标本的蛋白样品,其中NB和GNB患儿各7例,进行WB分析,发现NB和GNB中相对灰度值的中位数和四分位数间距分别是1.25(IQR:0.87~1.51)和0.24(IQR:0.20~0.30),差异有统计学意义(P=0.002),见图 2a,2b。这说明CDC5L在神经母细胞瘤不同病理类型存在差异表达,为进一步分析CDC5L和神经母细胞瘤患儿其它临床病理信息之间关系的研究奠定了基础。
|
Download:
|
| 图 2 肿瘤组织蛋白中CDC5L的表达情况 注 a:WB分析CDC5L在NB和GNB患儿中的表达;b:灰度定量分析CDC5L表达水平 Fig. 2 CDC5L expression in tumor tissue protein a:CDC5L expression in neuroblastoma tissue (NB) and ganglioneuroblastoma tissue (GNB) from NB patient specimens using Western blot; b:CDC5L bands were quantifed and shown in the bar chart after normalization | |
在临床特征方面,CDC5L的表达与骨髓浸润状态及临床分期有关,有骨髓浸润和临床分期Ⅲ~Ⅳ者CDC5L高表达的比例明显高于无骨髓浸润和临床分期Ⅰ~Ⅱ/Ⅳ-S者(P=0.016和P=0.001);危险度分级中,高危患儿中CDC5L高表达的比例较中危和低危患儿高(χ2=9.378,P=0.002);而不同性别和年龄段NB中CDC5L呈高表达的比例无统计学差异(P>0.05)。
病理特征方面,病理诊断为NB者CDC5L高表达的比例较GNB高(χ2=7.939,P=0.005);有MYCN扩增的患儿CDC5L呈高表达的比例较MYCN无扩增者更高(P=0.038);再按Shimada病理分型,将患儿分为良好组织学类型组和不良组织学类型组,同样表现为UFH组较FH组CDC5L高表达比例更高(χ2=7.839,P=0.005)。
实验室检查方面,CDC5L在NSE异常组中高表达的比例高于NSE正常组(P=0.035);CDC5L在LDH≥587 U/L组中高表达比例高于LDH<587 U/L组(χ2=5.547,P=0.019),见表 2。
|
|
表 2 CDC5L表达水平与NB患儿临床病理特征之间的相关性分析[n(%)] Table 2 Correlation between CDC5L expression and clinicopathological characteristics in NB patients[n(%)] |
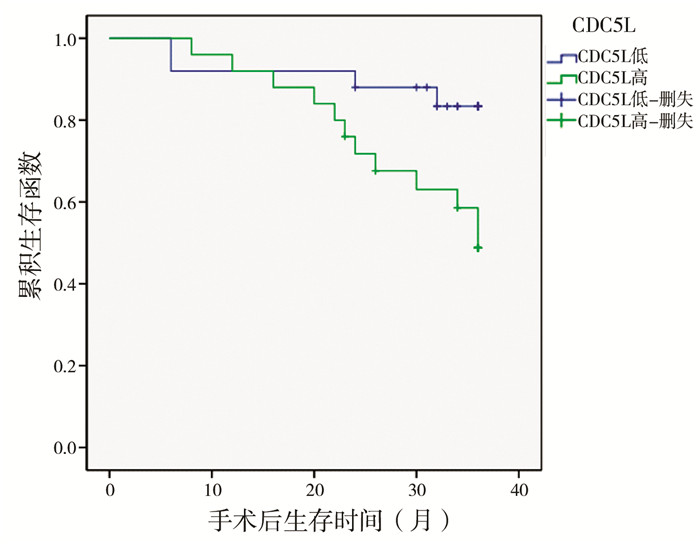
在不良病理类型、临床高分期及高危险度等已确定的患儿的不良预后因素中CDC5L高表达比例明显增高,这提示CDC5L的表达可能对患儿预后有影响。对50例随访信息完整的患儿进一步行Kaplan-Meier生存曲线分析,发现CDC5L高表达的患儿3年存活率(48.8% vs.88.0%)明显低于CDC5L低表达的患儿(P=0.002)。
单因素Cox回归分析发现CDC5L高表达(HR=4.734,95%CI:1.590~14.091)、临床分期为Ⅲ和Ⅳ期(HR=7.444,95%CI:2.187~25.339)、高危状态(HR=2.749,95%CI:1.454~5.194)、Shimada病理UFH型(HR=4.098,95%CI:1.493~11.250)均是患儿预后的危险因素。但是进一步多因素Cox回归分析,发现上述因素均无统计学差异(P>0.05),见表 3。
|
Download:
|
| 图 3 CDC5L在NB患儿中高表达和低表达的总体生存率比较 Fig. 3 Comparison of overall survival rate between high and low expression of cdc5l in children with NB | |
|
|
表 3 CDC5L表达、临床分期、危险度分级和Shimada病理分型对NB患儿死亡的单因素和多因素Cox回归分析 Table 3 Single variable and multivariable analysis of the effect of CDC5L expression, clinical stage, risk classification and Shimada pathological type on overall survival in NB patients |
神经母细胞瘤(neuroblastoma, NB)是一种高度异质性的肿瘤,部分患儿可以发生肿瘤自发性消退,而恶性程度高的患儿,即使经过多学科联合强化治疗后病情仍可进展,其高度异质性的特征和NB的临床和分子生物学行为密切相关[12, 13]。因此,对NB患儿的治疗也应个体化,规范化、个体化的综合治疗在过去60年间已得到明显提高。目前对儿童恶性实体瘤进行分子靶标检测的研究仍在进行,以期对儿童恶性实体瘤的个体化治疗提供更多循证医学依据[14, 15]。2009年国际NB危险组通过大规模的队列研究确定了包括年龄、肿瘤分期、组织学类型、分化程度、MYCN扩增状态等13个和NB预后相关的危险因素,为NB的危险分层和治疗措施的选择提供了参考依据[10]。近年来,随着人们对肿瘤发生及进展的分子机制及生物学行为的不断探索,与恶性肿瘤生物学行为相关的分子研究也不断涌现,CDC5L与肿瘤的危险程度及预后的研究逐渐成为目前研究的热点。目前研究初步揭示了CDC5L与多种肿瘤的不良预后有关[2, 16]。有研究表明CDC5L与Prp19一起参与前体mRNA的剪接、DNA的损伤修复和底物泛素化修饰等,对多种蛋白的生成和生物学作用的发挥起到关键作用[1]。CDC5L还被认为是一种细胞周期相关的蛋白,已有研究指出一些细胞周期进程中的重要调控蛋白在多种肿瘤中呈高表达,且其表达水平与肿瘤预后呈负相关[17, 18]。CDC5L的过度表达可促进细胞G2/M期转变,加速细胞有丝分裂进程,相反,抑制CDC5L的表达,则可阻滞细胞分裂进程,促进细胞凋亡和染色体的畸变[19-21]。这些特性决定了CDC5L的过度表达和肿瘤的不良预后[20, 22]。然而目前尚未见到儿童肿瘤的危险度和预后与CDC5L的相关报道。本文结合免疫组化和WB来探讨CDC5L对NB患儿临床、病理和预后的影响。
肿瘤的临床分期(INSS分期)是已经确立的对肿瘤患儿预后产生不良影响的因素,分期越高,患儿的预后越差。本研究同样发现Ⅲ~Ⅳ期患儿CDC5L呈高表达的比例明显高于Ⅰ~Ⅱ/Ⅳ-S期患儿。已有研究表明MYCN的扩增和NB的进展及预后密切相关,通常MYCN扩增预示着NB患儿恶性程度高,侵袭性强,预后差[10, 23-25];反之,在很多NB模型中,MYCN的下调可以逆转肿瘤干细胞表型和异常的侵袭能力[26, 27]。而本研究中CDC5L低表达组均无MYCN的扩增,这间接说明CDC5L低表达组NB恶性程度低,患儿预后较好。骨髓转移是NB最常见的转移部位,根据INSS分期系统,有骨髓转移者定义为Ⅳ期,对是否有骨髓转移进行分析,有骨髓转移者CDC5L表达增高的比例较无骨髓转移者高,这再次验证了CDC5L和高临床分期有关[28-30]。诊断年龄也是影响患儿预后的因素,诊断年龄<18个月者较>18个月者预后差,而本研究发现不同年龄组中CDC5L表达增高的比例没有差异,这说明年龄可能并不是影响CDC5L表达的因素。
通常认为,GNB的预后要好于NB[10, 29]。因此推测CDC5L在NB患儿中的表达高于GNB,本研究结果与假设相符,NB患儿中CDC5L呈高表达的比例高于GNB患儿。研究发现Shimada病理分型也是影响患儿预后的危险因素,良好组织学类型者预后优于不良组织学类型。按照Shimada病理分型标准对本研究中患儿进行分组,结果也显示UFH组中CDC5L表达增高的比例明显高于FH组[31]。这表明了CDC5L的表达和病理分类有较好的一致性,可作为病理分型的一个参考标准,尤其是肿瘤切除不完全时,可以为NB和GNB的区分提供参考。
神经元特异性烯醇化酶(NSE)是NB的敏感性和特异性标记物,乳酸脱氢酶(LDH)是肿瘤敏感而非特异的标记物,发生NB时通常伴有这些肿瘤标记物的升高,为NB的诊断提供参考[32]。另外,它们还可评估NB的治疗效果及预测NB的进展、复发及预后[10, 32-34]。在NB患儿中,NSE来源于肿瘤组织,与患儿的临床过程密切相关,可作为NB的诊断、预后和随访的参考标准之一。本研究发现,NSE的高表达和CDC5L的表达有较好的一致性,这提示CDC5L的表达对NB的诊断和预后评估有一定的参考价值。有学者将LDH水平以580 U/L或587 U/L为界值点将所有NB患儿分为两组,LDH水平高于580 U/L或587 U/L均是NB患儿不良预后的因素[10, 34]。本研究中也发现LDH水平高于587 U/L组的患儿中CDC5L呈高表达的比例高于LDH水平低于587 U/L组的患儿,且高的LDH水平和NB的不良预后有关(数据未显示)。因此,本研究认为CDC5L的表达水平可反映LDH的水平,从而间接反映NB患儿的预后,进一步对CDC5L对NB患儿生存的影响进行Log-lank检验,发现CDC5L高表达的患儿预后明显差于CDC5L低表达的患儿;Cox单因素回归分析再次证实这一结果,表现为CDC5L高表达是患儿预后不良的危险因素;除CDC5L外,临床高分期、高危状态、Shimada病理UFH型和骨髓浸润也都是NB患儿预后不良的危险因素。但将上述因素进一步行多因素Cox回归分析,发现以上因素均无统计学差异。原因可能是各因素之间存在交互作用,或样本量太小,显示不出统计学差异,需进一步增加样本量提高检验效能。
综上,CDC5L的高表达和NB患儿的临床高分期、MYCN扩增、不良组织学类型(UFH)及肿瘤标志物的升高均有很好的一致性,因此CDC5L表达升高可用于NB患儿的风险评估,并可为NB患儿临床分期、组织病理分类等提供参考。同时生存曲线分析及Cox回归单因素分析均表明CDC5L是NB患儿预后不良的危险因素,但CDC5L是否是NB患儿预后不良的独立危险因素,尚需进一步增大样本量进行分析验证。另外,我们也应积极探索CDC5L与NB恶性行为相关的分子机制,针对该机制达到对NB靶向治疗,至少是辅助治疗的目的。
| 1 |
Sihn CR, Cho SY, Lee JH, et al. Mouse homologue of yeast Prp19 interacts with mouse SUG1, the regulatory subunit of 26S proteasome[J]. Biochem Biophys Res Commun, 2007, 356(1): 175-180. DOI:10.1016/j.bbrc.2007.02.134. |
| 2 |
Jie Y, Lan W, Zhu JM, et al. Prp19 facilitates invasion of hepatocellular carcinoma via p38 mitogen-activated protein kinase/Twist1 pathway[J]. Oncotarget, 2016, 7(16): 21939-21951. DOI:10.18632/oncotarget.7877. |
| 3 |
Huang R, Xue R, Qu D, et al. Prp19 Arrests Cell Cycle via Cdc5L in Hepatocellular Carcinoma Cells[J]. Int J Mol Sci, 2017, 18(4): 778. DOI:10.3390/ijms18040778. |
| 4 |
Lu S, Wang R, Cai C, et al. Small Kinetochore Associated Protein (SKAP) Promotes UV-Induced Cell Apoptosis through Negatively Regulating Pre-mRNA Processing Factor 19 (Prp19)[J]. PLoS One, 2014, 9(4): e92712. DOI:10.1371/journal.pone.0092712. |
| 5 |
Bernstein HS, Coughlin SR. Pombe Cdc5-related protein.A putative human transcription factor implicated in mitogen-activated signaling[J]. J Biol Chem, 1997, 272(9): 5833-5837. DOI:10.1074/jbc.272.9.5833. |
| 6 |
Zhang N, Kaur R, Akhter S, et al. Cdc5L interacts with ATR and is required for the S-phase cell-cycle checkpoint[J]. Embo Reports, 2009, 10(9): 1029. DOI:10.1038/embor.2009.122. |
| 7 |
Boudrez A, Beullens M, Groenen P, et al. NIPP1-mediated interaction of protein phosphatase-1 with CDC5L, a regulator of pre-mRNA splicing and mitotic entry[J]. J Biol Chem, 2000, 275(33): 25411-25417. DOI:10.1074/jbc.M001676200. |
| 8 |
Sadikovic B, Thorner P, Chilton-Macneill S, et al. Expression analysis of genes associated with human osteosarcoma tumors shows correlation of RUNX2 overexpression with poor response to chemotherapy[J]. BMC Cancer, 2010, 10(1): 1-9. DOI:10.1186/1471-2407-10-202. |
| 9 |
Martin JW, Chiltonmacneill S, Koti M, et al. Digital Expression Profiling Identifies RUNX2, CDC5L, MDM2, RECQL4, and CDK4 as Potential Predictive Biomarkers for Neo-Adjuvant Chemotherapy Response in Paediatric Osteosarcoma[J]. PLoS One, 2014, 9(5): e95843. DOI:10.1371/journal.pone.0095843. |
| 10 |
Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system:an INRG Task Force report[J]. J Clin Oncol, 2009, 27(2): 289-297. DOI:10.1200/JCO.2008.16.6785. |
| 11 |
中国抗癌协会小儿肿瘤专业委员会. 儿童神经母细胞瘤诊疗专家共识[J]. 中华小儿外科杂志, 2015, 36(1): 3-7. DOI:10.3760/cma.j.issn.0253-3006.2015.01.00. Chinese Children Cancer Group. Expert consensus on diagnosis and treatment of neuroblastoma in Children[J]. Chin J Pediatr Surg, 2015, 36(1): 3-7. DOI:10.3760/cma.j.issn.0253-3006.2015.01.00. |
| 12 |
Brodeur GM. Neuroblastoma:biological insights into a clinical enigma[J]. Nature Reviews Cancer, 2003, 3(3): 203-216. DOI:10.1038/nrc1014. |
| 13 |
Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma[J]. Lancet, 2007, 369(9579): 2106-2120. DOI:10.1016/S0140-6736(07)60983-0. |
| 14 |
董岿然. 我国儿童胚胎性恶性实体肿瘤的研究和治疗进展[J]. 临床小儿外科杂志, 2017, 16(5): 417-421. DOI:10.3969/j.issn.1671-6353.2017.05.001. Dong KR. Diagnosis and treatment of embryonic malignant tumors in children in China[J]. J Clin Ped Sur, 2017, 16(5): 417-421. DOI:10.3969/j.issn.1671-6353.2017.05.001. |
| 15 |
李斯文, 王珊, 杨超, 等. 药物相关性分子靶标检测在儿童恶性实体肿瘤个体化治疗中的初步研究[J]. 临床小儿外科杂志, 2017, 16(4): 341-346. DOI:10.3969/j.issn.1671-6353.2017.004.008. Li SW, Wang S, Yang C, et al. Preliminary identification of drug-related genomic markers for individualized therapy in children with malignant solid tumors[J]. J Clin Ped Sur, 2017, 16(4): 341-346. DOI:10.3969/j.issn.1671-6353.2017.004.008. |
| 16 |
Qiu H, Zhang X, Ni W, et al. Expression and Clinical Role of Cdc5L as a Novel Cell Cycle Protein in Hepatocellular Carcinoma[J]. Dig Dis Sci, 2016, 61(3): 795-805. DOI:10.1007/s10620-015-3937-9. |
| 17 |
Chao WC, Kulkarni K, Zhang Z, et al. Structure of the mitotic checkpoint complex[J]. Nature, 2012, 484(7393): 208-213. DOI:10.1038/nature10896. |
| 18 |
Hanahan D, Weinberg RA. Hallmarks of cancer:the next generation[J]. Cell, 2011, 144(5): 646-674. DOI:10.1016/j.cell.2011.02.013. |
| 19 |
Qiu H, Zhang X, Ni W, et al. Expression and Clinical Role of Cdc5L as a Novel Cell Cycle Protein in Hepatocellular Carcinoma[J]. Dig Dis Sci, 2016, 61(3): 795-805. DOI:10.1007/s10620-015-3937-9. |
| 20 |
Chen W, Li Z, Yan W, et al. Expression of CDC5L is associated with tumor progression in gliomas[J]. Tumour Biol, 2016, 37(3): 4093-4103. DOI:10.1007/s13277-015-4088-5. |
| 21 |
Mu R, Wang YB, Wu M, et al. Depletion of pre-mRNA splicing factor Cdc5L inhibits mitotic progression and triggers mitotic catastrophe[J]. Cell Death Dis, 2014, 5(3): e1151. DOI:10.1038/cddis.2014.117. |
| 22 |
Mohammadi M, Goudarzi PK, Rahmani O, et al. Evaluation of gene expression level of CDC5L and MACC1 in poor prognosis and progression of osteosarcoma[J]. Tumor Biology, 2016, 37(6): 8153-8157. DOI:10.1007/s13277-015-4726-y. |
| 23 |
Brodeur GM, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage[J]. Science, 1984, 224(4653): 1121. DOI:10.1126/science.6719137. |
| 24 |
Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas[J]. N Engl J Med, 1985, 313(18): 1111-1116. DOI:10.1056/NEJM198510313131802. |
| 25 |
Zeid R, Lawlor MA, Poon E, et al. Enhancer invasion shapes MYCN-dependent transcriptional amplification in neuroblastoma[J]. Nat Genet, 2018, 50(4): 515-523. DOI:10.1038/s41588-018-0044-9. |
| 26 |
Kang JH, Rychahou PG, Ishola TA, et al. MYCN silencing induces differentiation and apoptosis in human neuroblastoma cells[J]. Biochem Biophys Res Commun, 2006, 351(1): 192-197. DOI:10.1016/j.bbrc.2006.10.020. |
| 27 |
Tonelli R, Purgato S, Camerin C, et al. Anti-gene peptide nucleic acid specifically inhibits MYCN expression in human neuroblastoma cells leading to cell growth inhibition and apoptosis[J]. Mol Cancer Ther, 2005, 4(5): 779-786. DOI:10.1158/1535-7163.MCT-04-0213. |
| 28 |
Dubois SG, Kalika Y, Lukens JN, et al. Metastatic sites in stage Ⅳ and ⅣS neuroblastoma correlate with age, tumor biology, and survival[J]. J Pediatr Hematol Oncol, 1999, 21(3): 181-189. DOI:10.1097/00043426-199905000-00005. |
| 29 |
De BB, Gambini C, Haupt R, et al. Retrospective study of childhood ganglioneuroma[J]. J Clin Oncol, 2008, 26(10): 1710-1716. DOI:10.1200/JCO.2006.08.8799. |
| 30 |
Cohen MD. International criteria for neuroblastoma diagnosis, staging, and response to treatment[J]. J Clin Oncol, 1994, 12(9): 1991-1993. DOI:10.1200/JCO.1994.12.9.1991. |
| 31 |
Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada system)[J]. Cancer, 1999, 86(2): 364-372. DOI:10.1002/(sici)1097-0142(19990715)86:2<364:aid-cncr21>3.0.co;2-7. |
| 32 |
Simon T, Hero B, Hunneman DH, et al. Tumour markers are poor predictors for relapse or progression in neuroblastoma[J]. Eur J Cancer, 2003, 39(13): 1899-1903. DOI:10.1016/S0959-8049(03)00376-9. |
| 33 |
Cangemi G, Reggiardo G, Barco S, et al. Prognostic value of ferritin, neuron-specific enolase, lactate dehydrogenase, and urinary and plasmatic catecholamine metabolites in children with neuroblastoma[J]. Onco Targets Ther, 2012, 5: 417-423. DOI:10.2147/OTT.S36366. |
| 34 |
London WB, Castel V, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma:a report from the International Neuroblastoma Risk Group project[J]. J Clin Oncol, 2011, 29(24): 3286-3292. DOI:10.1200/JCO.2010.34.3392. |
 2019, Vol. 18
2019, Vol. 18


